You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
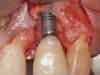
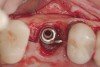
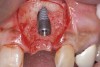
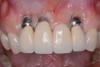
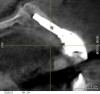
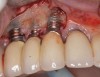
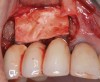
When dental implants fracture, they typically require removal. Implant fracture can lead to abutment instability and screw loosening, which may result in inflammation and infection as subgingival bacteria colonize normally inaccessible regions. This can lead to marginal and buccal/facial bone loss associated with the location of the fracture. When the coronal aspect of a restored implant fractures, it can cause mobility of the abutment and cemented restoration (Figure 1). In addition, multiple try-ins of ill-fitting restorations and torqueing of abutment screws can fracture an implant (Figure 2). Bone loss, swelling, and discomfort persisted after localized debridement and systemic antibiotic therapy; therefore, this implant was eventually removed.
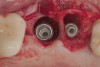
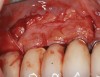
The challenge in removing implants fractured at the connection is primarily the difficulty in using reverse torque instrumentation. This method involves engaging the internal aspect of the implant to be removed. With a fractured implant, this method may cause further fracture, complicating its removal. Most commonly, these implants are removed with hollow, trephine drills. For a trephine to be effective, the inner diameter must be slightly larger than the widest portion of the fractured implant. This ensures that the trephine can move along the entire length of the implant without cutting into it. If the implant is sectioned at it's widest portion, which unfortunately results in significant spreading of titanium debris, a narrower trephine can be utilized to help conserve additional bone. After the threads of the implant body are no longer in contact with the surrounding bone, a small, root elevator is inserted circumferentially to fracture the apical portion of the fixture from the bone below. This is a very delicate procedure, especially when the implant is in close proximity to adjacent teeth or implants, the maxillary sinus, or the inferior alveolar canal. With proper diagnostics, which often include cone-beam computed tomography (CBCT) scans to evaluate the surrounding bone in three dimensions, this can be done safely. Figure 3 depicts an example of an implant that was removed using reverse torque instrumentation and one that was removed using a trephine.
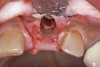
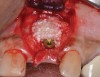
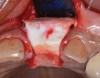
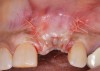
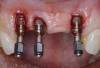
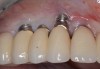
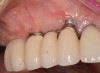
Because the osteotomy created by the trephine is wider than the originally placed implant, it may be challenging to immediately replace this implant unless there is sufficient apical bone available for implant stabilization. For some cases, a wider implant can be placed, but the physiologic rules requiring 1.5 to 2.0 mm for tooth/implant or 3.0 mm for implant/implant spacing,2 as well as other established esthetic criteria, must be respected. For example, a clinical examination revealed a fractured implant in the position of tooth No. 14 along with a fracture to tooth No. 13 (Figure 4). After trephine removal of the fractured implant and extraction of the fractured bicuspid, two implants were placed (Astra Tech, Dentsply Sirona), utilizing apical and axial bone in the apical third of the osteotomy and alveolus to stabilize both fixtures (Figure 5). When this cannot be performed, a staged approach should be employed wherein the site is augmented to facilitate implant placement 4 to 6 months after explantation and grafting.
Occasionally, when a fractured implant is removed via a trephine drill, a wider implant can be placed that will still be within the osseous envelope and respectful of physiologic and esthetic guidelines (Figure 6 through Figure 10). After explantation of an implant with a 3.6-mm diameter body that tapered to 2.5 mm apically, a coronally tapered 4.2-mm diameter implant with a 2.5-mm apical diameter (Astra Tech, Dentsply Sirona) was placed with adequate implant stability. The facial bone lost secondary to the fracture was augmented simultaneously with immediate implant replacement.
Malpositioned Implants
When implants are not placed in ideal 3-dimensional positions, hard- and soft-tissue remodeling often leads to abutment and/or thread exposure and esthetic failures (Figure 11 and Figure 12).3,4 When this occurs and implants are present "outside the bony envelope," it may be more predictable to remove these implants, augment the sites with hard and/or soft tissues, and replace the fixtures in more favorable positions. Under these circumstances, removing the intact implants without any surrounding bone is preferred. This can be achieved with reverse torque instrumentation. In a study evaluating palatal implants used for orthodontic anchorage, Kuhn and colleagues demonstrated that there are fewer complications associated with the reverse torque instrumentation method when compared with the older, trephine drill method.5
In the reverse torque method, high torque values of up to 200 Ncm are applied to the internal aspect of the implant to overcome the strength of the bone-to-implant contact (ie, osseointegration) and "fracture" the implant from it's osseous housing. The implants can then be easily rotated counterclockwise out of the site (Figure 13 and Figure 14). It is important to note that the strength of osseointegration may be significant and above the threshold of reverse torque devices. This can lead to fracture of the implant or the instruments used. If this occurs, it is usually necessary to utilize a trephine to complete the implant removal. For cases in which potential fracture during reverse torque instrumentation is anticipated, such as those involving wide, long, or hollow implants, many of the reverse torque kits contain thin trephine drills. These are intended to be used in the coronal aspect to separate the cortical bone at the crest, reducing the force necessary to reverse torque implants out of their osteotomies. Because these trephines are ultrathin, they are prone to fracture and are not meant to be used along the entire length of the fixture being removed. In addition, it is critical to only use these trephines in a vertical direction. Attempting to luxate an implant while it is inside the trephine will likely fracture the trephine. If this occurs, wider trephines or piezoelectric bone saws will be necessary to remove the retained portion of the fractured trephine. When reverse torque kits are used cautiously, bone can be preserved and immediate implant replacement can be considered.
Contraindications for Implant Removal
It is important to point out that not all implants presenting with mucogingival or esthetic complications require explantation, especially when the affected implants are partially supporting a multiple-unit restoration. This can be demonstrated in the following case. Implants were placed almost 20 years prior to the patient presenting with advanced soft-tissue recession on the maxillary right arch (Figure 15). There was a lack of keratinized/attached mucosa and buccal bone deficiency. This bone deficiency was not pathologic, but the result of physiologic remodeling,6 which was evident following flap reflection (Figure 16). At the time that these implants were placed, simultaneous bone augmentation to offset the diminution of the arch after tooth extractions was not a prerequisite.7 Also, the importance of soft-tissue augmentation at the time of immediate implant placement was not fully appreciated when this patient was originally treated.8,9 Formation of biologic width combined with a thin periodontal biotype often results in significant crestal bone loss.10 More recently, the importance of increasing soft-tissue thickness as it relates to more favorable crestal bone preservation has been demonstrated by Linkevicius and colleagues.11 Removing these implants would commit the patient to remaking her large prosthesis; therefore, she was interested in maintaining them with a corrective mucogingival procedure.
After gentle debridement with glycine air abrasion and sterile saline, a cross-linked, porcine collagen bone matrix (OSSIX® VOLUMAX; Datum Dental Ltd.) was placed over the buccal aspect of all three affected implants (Figure 17). Next, a subepithelial, connective tissue graft harvested from the right aspect of the patient's hard palate was affixed over the bone scaffold and exposed implants and abutments (Figure 18). And finally, a coronally advanced flap was sutured over the hard- and soft-tissue grafts. The 1-month, follow-up photograph demonstrates incomplete, but significant coverage of the previously exposed implants and abutments (Figure 19).
Due the loss of crestal bone in 360 degrees around these implant platforms, it is unrealistic to anticipate complete coverage of the implant abutments and collars.12 This procedure arrested the progress of recession, regenerated some of the lost facial bone, and increased the zone of keratinized mucosa.
Discussion
At the inception of implant therapy more than 30 years ago, achieving osseointegration was considered the end point of treatment. This was a "bone-driven" discipline, in which patients were deemed appropriate candidates if they presented to the surgical specialist with adequate bone volume to place root-form implants. Knowledge regarding the long-term behavior of hard and soft tissues after extractions and implant placement was incomplete. This resulted in the placement of implants with inadequate diameters for non-axial loading. Hence, innovations such as custom abutments, cement-retained restorations, and pink ceramics often saved otherwise non-restorable patients. As the literature attempted to answer the questions posed by clinicians after complications arose, the physiologic remodeling of bone after implant placement became better understood.13,14 The placement of wide diameter implants to purposely obturate entire extraction sockets and replace posterior teeth without consideration of the long-term complications has led to the ultimate failure of many implant procedures.15 Although it has been demonstrated that osseointegration can still occur with a gap present between the socket walls and implant surface,16 it may not be prudent to leave this gap unfilled.7 Currently, implant diameters are selected that intentionally result in a gap between the facial socket wall and the implant,17 and techniques to manage this space vary, as do opinions regarding the most appropriate grafting materials. Research has demonstrated that the use of regenerative therapy can improve esthetic outcomes when compared with those of ungrafted sites.18,19
Today, implant therapy is now a prosthetic- or occlusally-driven discipline.20 Working from the "top down," starting with the digital version of the final prosthesis and merging DICOM and STL files to visualize the hard and soft tissues as they relate to the anticipated restoration prior to treatment has forever changed implant therapy. The need for augmentation to facilitate axial implant placement and prevent ridge resorption and esthetic compromise can now be identified before the clinician even picks up an instrument.
Software that allows surgeons and restorative dentists to educate their patients about their individual circumstances before treatment begins minimizes the risks of miscommunication and unrealistic expectations. As patients treated in the earlier days of implant therapy (or by poorly trained dentists who were unaware of the long-term ramifications of their treatments) present for remediation of failing implant therapy, the need to be able to conservatively remove implants is critical. By removing implants that cannot be predictably restored to an appropriate state of health, function, and esthetics, patients are given another opportunity to experience a successful outcome.
Conclusion
As with any therapy, proper diagnosis, prognosis, and treatment planning are critical for patients requiring implant removal. All members of the team (ie, restorative, surgical, laboratory) must be involved prior to the commencement of treatment. When implants are being explanted, the end result must be visualized, accepted, and serve as the goal. How patients will be provisionalized, which team members will perform specific steps, and the costs associated with treatment must be discussed in advance as well. Patients should be presented with all available treatment options and managed to maintain realistic expectations regarding outcomes. With newer instrumentation methods, implant removal can be less invasive-reducing morbidity and decreasing treatment time.
About the Author
Barry P. Levin, DMD
Diplomate
American Board of Periodontology
Clinical Associate Professor
University of Pennsylvania
Philadelphia, Pennsylvania
Private Practice
Jenkintown, Pennsylvania
References
1. Tarnow DP, Chu SJ, Fletcher PD. Clinical decisions: determining when to save or remove an ailing implant. Compend Contin Educ Dent. 2016;37(4):233-243.
2. Kois JC. Predictable single-tooth peri-implant esthetics: five diagnostic keys. Compend Contin Educ Dent. 2001;22(3):199-206.
3. Chen ST, Darby IB, Reynolds EC, et al. Immediate implant placement postextraction without flap elevation. J Periodontol. 2009;80(1):163-172.
4. Peng M, Fei W, Hosseini M, et al. Influence of implant position on clinical crown length and peri-implant soft tissue dimensions at implant-supported single crowns replacing maxillary central incisors. Int J Periodontics Restorative Dent. 2013;33(6):785-793.
5. Kuhn M, Göllner P, Schätzle M, et al. Non-invasive removal of sandblasted and acid-etched titanium palatal implants, a retrospective study. Eur J of Orthod. 2015;37(6):584-588.
6. Roe P, Kan JY, Rungcharassaeng K, et al. Horizontal and vertical dimensional changes of peri-implant facial bone following immediate placement and provisionalization of maxillary anterior single implants: a 1-year cone beam computed tomography study. Int J Oral Maxillofac Implants. 2012;27(2):393-400.
7. Kan JY, Rungcharassaeng K, Lozada JL, et al. Facial gingival tissue stability following immediate placement and provisionalization of maxillary anterior single implants: a 2- to 8- year follow-up. Int J Oral Maxillofac Implants. 2011;26(1):179-187.
8. Grunder U. Crestal ridge width changes when placing implants at the time of tooth extraction with and without soft tissue augmentation after a healing period of 6 months: report of 24 consecutive cases. Int J Periodontics Restorative Dent. 2011;31(1):9-17.
9. Yoshino S, Kan JY, Rungcharassaeng K, et al. Effects of connective tissue grafting on the facial gingival level following single immediate implants placement and provisionalization in the esthetic zone: a 1-year randomized controlled prospective study. Int J Oral Maxillofac Implants. 2014;29(2):431-440.
10. Berglundh T, Lindhe J. Dimension of the periimplant mucosa. Biological width revisited. J Clin Periodontol. 1996;23(10):971-973.
11. Linkevicius T, Puisys A, Linkeviciene L, et al. Crestal bone stability around implants with horizontally matching connection after soft tissue thickening: a prospective clinical trial. Clin Implants Dent Relat Res. 2015;17(3):497-508.
12. Miller PD Jr. A classification of marginal tissue recession. Int J Periodontics Restorative Dent. 1985;5(2):8-13.
13. Botticelli D, Berglundh T, Lindhe J. Hard-tissue alterations following immediate implant placement in extraction sites. J Clin Periodontol. 2004;31(10):820-828.
14. Botticelli D, Renzi A, Lindhe J, et al. Implants in fresh extraction sockets: a prospective 5-year follow-up clinical study. Clin Oral Implants Res. 2008;19(12):1226-1232.
15. Buser D, Chappuis V, Belser UC, et al. Implant placement post extraction in esthetic single tooth sites: when immediate, when early, when late? Periodontology 2000. 2017;73(1):84-102.
16. Wilson TG Jr, Schenk R, Buser D, et al. Implants placed in immediate extraction sites: a report of histologic and histometric analyses of human biopsies. Int J Oral Maxillofac Implants. 1998;13(3):333-341.
17. Capelli M, Testori T, Galli F, et al. Implant-buccal plate distance as diagnostic parameter: a prospective cohort study on implant placement in fresh extraction sockets. J Periodontol. 2013;84(12):1768-1774.
18. Tarnow DP, Chu SJ, Salama MA, et al. Flapless postextraction socket implant placement in the esthetic zone: part 1. The effect of bone grafting and/or provisional restoration on facial-palatal ridge dimensional change-a rettrospective cohort study. Int J Periodontics Restorative Dent.2014;34(3):323-331.
19. Levin BP. The dermal apron technique for immediate implant socket management: a novel technique. J Esthet Restor Dent. 2016;28(1):18-28.
20. Garber DA, Belser UC. Restoration-driven implant placement with restoration-generated site development. Compend Contin Educ Dent. 1995;16(8):796-804.