You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
The history of dental adhesives can be viewed from a generational perspective, starting with the first generation and moving forward to the current state of the art. In the 1960s, etching was done only on the enamel to improve the interface between the resin and the tooth. In the 1970s, etching was still restricted to the enamel, but it was accomplished with better chemistry that resulted in better bond strength. In the 1980s, the etch-and-rinse concept known as total-etch was introduced. This involved three steps: etching, application of primer, and, finally, application of the adhesive. In the 1990s, the total-etch process evolved to use a combined primer and adhesive. This reduced the number of steps to two: etching followed by application of the primer/adhesive combination. In the 2000s, these two steps were reduced to one.
More recently, “universal” or “multimode systems” have been introduced. These still combine the etch, primer, and adhesive, but they can be used in three different application modes. The dictionary defines universal as covering all or a whole collectively, without limit or exception. By definition, a universal adhesive should produce the same results no matter which application technique is used. It should be capable of being used with other brands of cement without having to add activators or change the technique.
Thus, these questions can be posed: are universal adhesives truly universal? Can all universal adhesives be used without limit or exception? Are there exceptions depending on the conditions? Is it possible that manufacturers have a different understanding or definition of the word “universal”? This article examines those questions, in particular focusing on the different application modes and the most predictable bonding techniques.
A substantial number of universal adhesives currently are on the market, including:
• Brush&Bond® (Parkell; www.parkell.com)
• Scotchbond™ Universal (3M ESPE; www.3m.com)
• Futurabond® U (Voco; www.voco.com)
• CLEARFIL™ Universal Bond (Kuraray; www.kuraraydental.com)
• ALL-BOND UNIVERSAL (Bisco; www.bisco.com)
• Peak® Universal (Ultradent; www.ultradent.com)
• Adhese® Universal (Ivoclar Vivadent; www.ivoclarvivadent.us)
• Optibond™ XTR (Kerr; www.kerrdental.com)
• Prime&Bond Elect® (Dentsply; www.primeandbondelect.com)
All advocate multiple modes of application. They can be used in a one-step, self-etch mode or in a two-step, etch-and-rinse process. As a third alternative, the etching can be done selectively, affecting only the enamel. Two steps are nonetheless still required.
The major universal adhesive functional monomers are:
• 10-methacryloyloxydecyl dihydrogen phosphate (10-MDP)
• 4-methacryloyloxyethyl trimellitate anhydride (4-Meta)
• Glycerol-phosphate dimethacrylate (GPDM)
• 2-methacryloxyethyl phenyl phosphate (Phenyl-P)
• 4-methacryloyloxyethyl trimellitate acid (4-Met)
• Methacryloyloxy dodecylpyridinium bromide (MDPB)
Dentistry’s understanding of how functional monomers interact with hydroxyapatite was advanced by the adhesion-decalcification concept developed by Yoshida in 2001.1 This concept suggests that minerals are removed from tooth structure and replaced with resin monomers in an exchange process. Historically, adhesive bonding has involved a micromechanical interlocking of monomers to porosities that are created by various means. Certain adhesive monomers can also chemically bond to the calcium in hydroxyapatite. Depending on the type of monomer, the phosphate or carboxyl group will bond to the hydroxyapatite, and one of two things will occur.2
In the first scenario, ionic interaction occurs with only a limited amount of decalcification. The limited amount of decalcification allows hydroxyapatite to remain and creates some porosities for micromechanical retention. A chemical reaction occurs between the functional monomer and the remaining calcium, creating a monomer-calcium salt. This is what happens in the self-etch mode.
The second scenario involves more decalcification without any chemical adhesion; most of the collagen is exposed. In this event, 37% phosphoric acid is electron-negative and is attracted to the positively charged calcium ion, where it is removed from the tooth surface, causing major decalcification. This describes the etch-and-rinse mode.
The most commonly used functional monomers are 10-MDP and 4-Meta. Both are bifunctional monomers. Both have a methacrylate group on one end, and both have spacers between the two ends. However, the MDP monomer has a phosphate-ester group on its second end, while 4-Meta has a carboxylic group. The spacers also differ, with the MDP spacer consisting of a 10-carbon chain that is a much larger molecule. The 4-Meta spacer is a 2-carbon chain.
10-MDP
The 10-MDP functional monomer works as follows. The hydrophobic methacrylate group reacts with monomers in resin materials having a methacrylate base. The hydrophilic phosphate group ionically bonds to tooth structures as well as zirconia and metal oxides. The 10-hydrocarbon chain is a balance between the hydrophobic and hydrophilic end. The different ends align themselves in a process called self-assembly; the molecules spontaneously adopt a defined arrangement without any outside source of guidance. Somehow they find their way, align themselves, and self-assemble.
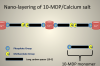
Self-assembled nanolayered structures have been reported in the resin–dentin interfaces created by adhesives containing 10-MDP. (Nanolayering is defined by the molecular lamination from a chemical interaction of functional groups that exists on the surface of a substrate material.) The nanolayered structures contribute to bond durability.3 Figure 1 illustrates the formation of the MDP calcium salt. In the interfacial nanolayering, the calcium-rich layer can be seen, along with the 10-MDP monomers, the methacrylate groups, and the phosphate groups. The phosphate groups attach to the calcium, and the methacrylate groups attach to themselves. This results in the self-assembled nanolayering, forming the 10-MDP-calcium salt.2
At the adhesive interface, the combination of 2-MDP molecules united by a calcium-MDP salt is the most resistant monomer to degradation over time. Phenyl-P, a precursor to 10-MDP, is not at all stable, and 4-Meta is in between.3 To determine whether nanolayering was produced by all universal adhesives, Tian et al examined seven commercial products, six of which contained HEMA.4 HEMA is commonly found in adhesives to improve wetting of the dentin and prevent phase separation of the resin components after solvent evaporation. Some studies have shown that the HEMA interferes with 10-MDP calcium nano-layering formation,5 while other studies have shown that it does not.6 Tian created three experimental versions of 10-MDP monomer: 5%, 10%, and 15%. All materials were used in a self-etch mode. Analysis was done by transmission electron microscopy (TEM) and x-ray diffraction. The researchers found that application of 15% 10-MDP experimental primer with no additional resinous components to coronal dentin in the self-etching mode resulted in profuse nanolayering in the resin-dentin interface. The 10-MDP found in CLEARFIL SE™ Bond (Kuraray) had limited nanolayering, and no nanolayering was found in the 10-MDP Scotchbond Universal.
Kuraray originally synthesized 10-MDP and obtained a patent for it that expired in 2011. However, the patent literature did not describe the synthesis and purification processes. Those processes are still under active patent protection until 2022.7 After 2011, many companies launched 10-MDP-based adhesives that they referred to as universal adhesives. However, the bonding effectiveness of various commercial 10-MDP-based adhesives has been found to vary,8 with the bond strengths dependent on the 10-MDP concentration.9
The pH levels of various 10-MDP monomers also vary. The pH of universal functional monomers also has been described as the etching aggressiveness. A very strong acid has a pH less than 1, whereas an ultra-mild acid has one greater than 2.5.10
A correlation has been found between pH and the compatibility of universal adhesives and self-cure and dual-cure cements. The more acidic the adhesive, the less compatible it will be. The acid deactivates the aromatic tertiary amines that are the base, which is critical to the chemical-curing portion of the cement. This is more of a problem when mixing different manufacturers’ adhesives and cements. Some manufacturers will supply a separate activator. Less acidic adhesives (around 3.2 pH) do not have this problem. Amine-free cements can also be used. The adhesive layer may need to be light-cured before placing the cement.
Application Modes for MDP-based Adhesives
The first application mode for MDP-based adhesives is the self-etching mode, in which the adhesive is applied to the prepared enamel and dentin simultaneously. The second mode is the etch-and-rinse (formerly known as total-etch) mode. In this, 35% phosphoric acid is applied to the entire prepared tooth surface (enamel and dentin) and then rinsed off. The adhesive is then applied. In the third mode, selective etch, 35% phosphoric acid is applied to the enamel only. The dentin remains unconditioned. The adhesive is then applied to the enamel and the dentin. However, isolating the dentin from contact with the acid can be difficult.
Although it might seem logical that the bond strengths of universal adhesives would be the same for the three different application modes, they are not.11 Initial data on bond strengths reflects studies conducted on sound dentin but, in clinical practice, the bonding substrate is usually caries-affected dentin, which has a different mineral content than sound dentin.12,13 The caries-affected dentin has greater porosity, allowing deeper penetration of the etchant and resulting in deeper layers of demineralization. It can be difficult for the resin to reach that depth, and a greater amount of unprotected collagen fibers are present in the hybrid layer.
In the self-etch mode, the high bond strength of the 10-MDP to dentin comes from the low dissolution rate of the calcium salt due to the high chemical bonding capability.14 The interaction of the 10-MDP with enamel is less than with hydroxyapatite and dentin. The structure and size of the hydroxyapatite crystals in enamel interfere with the chemical bonding to 10-MDP because they are much larger than the dentin crystals.9 Enamel acid-etching increases the bonding area and wettability of the adherent surface, promoting good micromechanical retention.15 Enamel bond strength using the self-etch mode is significantly lower compared to etched enamel.
In the etch mode, the enamel and dentin surfaces are etched simultaneously. This creates a deeper etch pattern on the enamel resulting in a greater bond strength.16 It increases the enamel porosity and results in greater resin interlocking and micromechanical retention.15 However, the combined negative effects of dentin acid-etching can include overetching, suboptimal removal of the acid, and incomplete adhesive infiltration into the denuded collagen network.17 This compromises the chemical adhesion by removing the residual hydroxyapatite from the collagen mesh,18 which in turn lowers the bond strength due to the decreased amount of hydroxyapatite available for chemical interaction with the functional monomer.19 This is monomer-dependent.
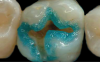
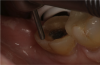
The third mode, selective-etch, is a combination of the first two modes, etching only the enamel and leaving the smear layer intact on the dentin, after which the universal adhesive is applied. This mode results in a better enamel etch pattern and allows for more 10-MDP calcium salt formation. The drawback is that it is very difficult to etch only the enamel without spillage onto the dentin (Figure 2). Even rinsing the etchant from the enamel will cause it to contact the dentin.
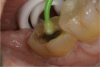
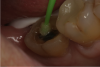
An alternative to the standard selective-etch technique is to apply the universal adhesive to both the enamel and dentin after the tooth is prepared but before the impression or scan. Active application of the adhesive (Figure 3) has a number of advantages. Agitation with a microbrush continuously keeps the fresh self-etchant in contact with the dentin, increases the rate of solvent evaporation, and facilitates fusion of the monomer into the smear layer. Manual pressure of the microbrush also compresses the collagen network. When the agitation is stopped and the pressure is relieved, the compressed collagen expands like a sponge and draws in the surrounding adhesive.20 After actively applying the 10-MDP-based adhesive, it is air-thinned to remove the remaining solvent and light-cured.
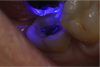
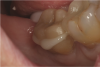
If a temporary restoration is being used, and two appointments are necessary, then the oxygen-inhibited layer should be removed by using an alcohol wipe or applying surgical lubricant over the tooth. Light-curing will then eliminate the oxygen-inhibited layer (Figure 4). This will also prevent any resin-based temporary material from sticking to the tooth. This is not necessary, however, if a same-day restoration created with a chairside milling machine is being delivered.
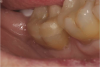
After light-curing, the enamel margin is then re-prepared with a fine end-cutting diamond bur to remove impregnated adhesive and expose the freshly cut enamel (Figure 5). Controlling the bur is easier than controlling the flow of phosphoric acid.
At the insertion phase, the entire preparation is acid-etched (Figure 6). This provides a deep etch pattern in the enamel and also cleans the resin-coated dentin. Universal adhesive is then applied to the entire preparation, and depending on the manufacturer’s instructions, light-cured or not. The restoration is then inserted and light-cured for few seconds, first on the buccal side and then on the lingual, while holding the restoration in place (Figure 7). Floss is inserted through the contacts, and any excess adhesive is removed before final light-curing (Figure 8). This alternative selective-etch technique provides immediate dentin sealing. It creates better self-etch dentin adhesion because it does not deplete the available calcium. It produces a better etch-and-rinse enamel etch pattern without accidental dentin demineralization, and it can be used for direct or indirect restorations.
4-Meta
The 4-Meta functional monomer is a diffusion-promoting monomer with two different functional ends, methacrylate and carboxylic. The hydrophobic methacrylate end has an affinity for methacrylate-based resins. The hydrophilic carboxylic end has an affinity for the dentinal surface-forming calcium salts. Functional monomers all have particular adhesion strategies. With the 4-Meta, the greater dentin bond strength is primarily micromechanical and only partially chemical; this is different from the 10-MDP-based adhesives. The greater enamel bond strength is micromechanical, the same as with 10-MDP-based adhesives. The 4-Meta adhesives are more acidic, smaller in molecular size, and have lower viscosity. Thus, they will penetrate deeper into the dentin and create a more intrinsic or extensive resin hybrid tag configuration, even without acid-etch, than the popular commercial universal resin used in the self-etch mode. For example, according to the manufacturer, the original Brush&Bond resin tag formation creates a better bond strength with a 3-µm film thickness because of the low viscosity.
Very few universal adhesives are dual-cure, which is important for deep areas inaccessible to light-activation. An additional co-initiator has been added to the universal adhesive. This increases the cross-linking between the polymer chains, which, in turn, increases durability.
When using the self-etch mode for a 4-Meta adhesive, the acidity level will provide good enamel and dentin penetration. The etch-and-rinse mode will provide greater bond strength and more stain resistance at the margins. Due to the acidity of 4-Meta, the selective-etch mode is not as advantageous as with 10-MDP-based adhesives. For application, agitation or rubbing is not recommended because this can collapse the more demineralized collagen network that is formed.
Conclusion
To summarize, universal adhesives are not as universal as advertised. Functional monomers differ in their type, as well as purity, pH levels, compatibility with various cements, and bond strengths in different modes of application. The bond strength is important, but the durability is equally important. No one technique or protocol fits all universal adhesives; it is essential to read the instructions that come with each product.
About the Author
Dr. Helvey is an adjunct associate professor at the Virginia Commonwealth University School of Dentistry. He also teaches cosmetic dentistry in the AEGD residency program and maintains a private practice in Middleburg, Virginia. He is the Restorative Section Editor and a member of the Editorial Advisory Board for Inside Dentistry.
References
1. Yoshida Y, Van Meerbeek B, Nakayama Y, et al. Adhesion to and decalcification of hydroxyapatite by carboxylic acids. J Dent Res. 2001;80(6):1565-1569.
2. Van Meerbeek B, Yohihara K, Yoshida Y, et al. State of the art of self-etch adhesives. Dent Mater. 2011;27(1):17-28.
3. Yoshida Y, Yoshihara K, Nagaoka N, et al. Self-assembled nano-layering at the adhesive interface. J Dent Res. 2012;91:376-381.
4. Tian F, Zhou L, Zhang Z, et al. Paucity of nanolayering in resin-dentin interfaces of MDP-based adhesives. J Dent Res. 2016;95(4):380-387.
5. Yoshida Y, Yoshihara K, Hayakawa S, et al. HEMA inhibits interfacial nano-layering of the functional monomer MDP. J Dent Res. 2012;91(11):1060-1065.
6. Yoshihara K, Yoshida Y, Nagaoka N, et al. Adhesive interfacial interaction affected by different carbon-chain monomers. Dent Mater. 2013;29(8):888-897.
7. Okada K, Otsuki J, Takahashi K, et al, inventors; Kuraray Co., Ltd., assignee. 2002 Sep3. Organophosphorus compounds for dental polymerisable compositions. United States patent US 6,458,868.
8. Wagner A, Wendler M, Petschelt A, et al. Bonding performance of universal adhesives in different etching modes. J Dent. 2014;42(7):800-807.
9. Yoshihara K, Yoshida Y, Hayakawa S, et al. Nano-layering of phosphoric acid ester monomer on enamel and dentin. Acta Biomater. 2011;7(8):3187-3195.
10. Perdigão J, Lopes MM, Gomes G. In vitro bonding performance of self-etch adhesives: II – ultramorphological evaluation. Oper Dent. 2008;33(5):534-549.
11. Banerjee A, Kellow S, Mannocci F, et al. An in vitro evaluation of microtensile bond strengths of two adhesive bonding agents to residual dentine after caries removal using three excavation techniques. J Dent. 2010;38(6):480-489.
12. Nakajima M, Kunawarote S, Prasansuuporn Y, et al. Bonding to caries-affected dentin. Japanese Dent Sci Rev. 2011;47(2):102-114.
13. Wang Y, Spencer P, Walker MP. Chemical profile of adhesive/caries-affected dentin interfaces using Raman microspectroscopy. J Biomed Mater Res A. 2007;81:(2):279-286.
14. Van Landuyt KL, Yoshia Y, Hirata I, et al. Influence of the chemical structure of functional monomers on their adhesive performance. J Dent Res. 2008;87(8):757-761.
15. Torii Y, Itou K, Hikasa R, et al. Enamel tensile bond strength and morphology of resin-enamel interface created by acid etching system with or without moisture and self-priming system. J Oral Rehabil. 2002;29(6):528-533.
16. Perdigão J, Sezinando A, Monteiro P. Evaluation of a new universal adhesive using different bonding strategies [abstract 18]. J Dent Res. 2012;91(special issue A).
17. Moszner N, Salz U, Zimmermann J. Chemical aspects of self-etching enamel-dentin adhesives: a systematic review. Dent Mater. 2005;21(10):895-910.
18. Yoshida Y, Nagakane K, Fukuda R, et al. Comparative study on adhesive performance of functional monomers. J Dent Res. 2004;83(6):454-458.
19. Van Landuyt KL, Kanumilli P, De Munck J, et al. Bond strength of a mild self-etch adhesive with and without prior acid-etching. J Dent. 2006;34(1):77-85.
20. do Amaral RC, Stanislawczuk R, Zander-Grande C, et al. Active application improves the bonding performance of self-etch adhesives to dentin. J Dent. 2009;37(1):82-90.