You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Management of alveolar ridge defects can present significant biologic and esthetic challenges for optimal implant placement when attempting to achieve a natural implant restoration outcome. The alveolar bone serves two functions for the implant: biologic, by housing the implant for osseointegration; and esthetic, by supporting the overlying peri-implant soft-tissue architecture, ultimately permitting the presentation of soft-tissue contours. Furhauser et al devised a pink esthetic score to evaluate esthetic success around single tooth implants.1 A careful study of their criteria indicates high esthetic success will be met when the implant is completely housed in bone and the crest is in a defined relationship to the papillary tips and facial-free gingival margin. These relationships have been established and quantified for natural teeth2-8 and implants.9-18 Proper management of ridge defects allows the clinician to achieve a higher pink esthetic score and, hence, more natural gingival contours for implant restorations for esthetics and stability of the peri-implant topography.19
Dentoalveolar ridge defects can arise from failure of the alveolar bone to form properly or by destruction of the existing alveolar bone. Failure of the alveolar bone to form has two local causes: congenital tooth absence, which inhibits alveolar bone growth, or ankylosis of a tooth before completion of facial growth,20-22 which arrests further alveolar bone growth. Destruction of existing alveolar bone has three causes: infection, trauma, or tooth loss. Infection can be from root fracture, endodontic origin, periodontitis, or peri-implantitis.
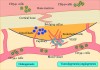
Defects that are of critical size do not spontaneously regenerate following injury, without adjunctive measures.23 They are recognized as having innately limited potential for alveolar regeneration and are required to be managed through bone regeneration techniques.24 When large dentoalveolar ridge defects exist, reestablishing a proper dentoalveolar ridge contour for an implant can be challenging, regardless of whether the defect is horizontal, vertical, or a combination.25 Predictable bone regeneration is dependent on four major biologic principles: primary wound closure, blood supply, space maintenance, and wound stability.26 The source of new bone is dependent on adequate blood supply at the site. The blood supply provides the necessary cells, growth factors, and inhibitors to initiate the osteogenic-biomineralization cascade. Osteocytes arise from stromal cells of the bone marrow and precursor cells that travel with capillary walls or circulate in the blood.27 Angiogenesis involves the formation of new capillaries that grow into a defect. The key to bone formation is promoting angiogenesis. The source of these new capillaries is from the remaining walls adjacent to the defect. Bone grafts maintain the volume of space of the defect, initiate bone resorption through inflammation, supply growth factors that are released as the graft is resorbed, and act as a chemo-attractant for angiogenesis. Bone-grafting success is most predictable when healing occurs in a contained environment (ie, primary intention wound healing is ensured) and new capillaries are allowed to fully vascularize the reconstruction.28-30 In general, horizontal alveolar bone defects are more predictable to treat because more walls (sources) are present for capillary in-growth into the osseous defect that is being reconstructed. Therefore, the capillaries have less distance to migrate for full infiltration of the defect. Vertical defects are less predictable to reconstruct because greater demands are placed on capillary migration and in-growth. The further the distance the capillaries must travel, the more difficult it is to achieve complete vascularization of the defect (Figure 1).
Distraction osteogenesis is a newer modality of guided bone regeneration (GBR) and has been used to help rebuild severe ridge defects.31 Orthodontics essentially is a form of distraction osteogenesis. Such therapy has been a longstanding modality of periodontal-orthodontic treatment to reduce or eliminate infrabony defects around teeth.32 Using distraction osteogenesis via tooth movement for GBR allows the clinician greater latitude in determining the size of the osseous defect to correct. Through tooth movement, the clinician can minimize the size and extent of the dentoalveolar ridge defect and have more predictable bone grafting. The smaller the defect, the more predictable the regenerative result will be.
The following case study illustrates the concept of orthodontically directed site development to optimize interdisciplinary implant dentistry.
Case Report
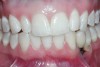
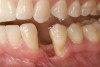
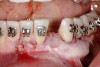
A 28-year-old Caucasian female presented to the periodontist with a complaint of a mobile implant in the site of tooth No. 22 (Figure 2 and Figure 3). The patient had a congenitally missing tooth No. 23. No. 22 had erupted into site No. 23, leaving a vacancy at site No. 22, and was the reason for the original implant at site No. 22.
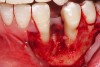
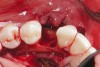
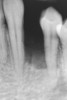
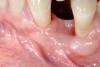
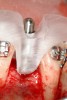
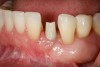
The patient had no medical concerns and had a history of an implant placement in December 2002 when she was 25 years old. In February 2005, she presented with a mobile implant and an associated advanced peri-implantitis. Radiographic review at the initial examination demonstrated significant destruction of the dentoalveolar ridge around the implant as well as around the adjacent natural dentition. Emergency-based treatment involved surgical implant removal only and debridement of the infection (Figure 4 and Figure 5). Following uneventful healing, an advanced ridge defect was apparent at the edentulous site and moderate and advanced attachment loss noted at No. 22D and No. 21M, respectively (Figure 6 and Figure 7). This case demonstrates bone loss of two separate origins: lack of bone because of tooth agenesis and destruction of bone from inflammatory peri-implantitis.
Because of the apparent bone loss, seen on the radiograph, on the mesial of tooth No. 21 and the distal of tooth No. 22, one suggestion was to remove teeth Nos. 21 and 22 and, subsequently, site-develop the regional anatomy for implant placement. The surface area requiring GBR was expected to be rather large, whereby autogenous bone grafting from the chin would have been elected to augment the site in both the horizontal and vertical dimensions. Following 6 months of healing, implant placement would ensue at either position, Nos. 21 or 23, to be restored as a bridge or at position Nos. 21 through 23 for individual implant restorations.
This approach incurred significant risk and inherent difficulty in meeting the biologic and esthetic goals of the patient. To recreate ideal soft-tissue contours, the graft would have to reach a vertical height that supports the papillary tips at a level as coronal as the papillary tips on the adjacent natural teeth (not to the level of the free gingival margin on the facial of the teeth). A vertical augmentation of this magnitude is the most difficult to achieve and has the least predictability. Autogenous block grafts are highly successful but do possess inherent challenges that include, but are not limited to, ensuring primary closure, minimizing infection during healing, ensuring graft stabilization and intimacy of fit during surgery, and having paresthesia potential from the operation. From an esthetic perspective, three implants placed adjacently pose a challenge for maintaining papillary soft-tissue form between implants.34 Two implants would be less challenging in this regard; however, the requirements for pontic site development would remain and require multiple periodontal plastic surgical procedures. In either option, optimal esthetic, biologic, occlusal, and biomechanical outcomes are at a compromise compared with a healthy natural dentition for the patient from a long-term predictability standpoint.
Another suggestion was to minimize the extent of the defect by moving the teeth orthodontically into the bony defect. First, tooth No. 22 would be returned to its proper functional and anatomic position. This approach would reduce the bony defect from the mesial aspect by the volume of one tooth. To reduce the defect from the distal aspect, tooth No. 21 would be extruded and extracted. To further reduce the defect, tooth No. 22 would be moved distally into site No. 21. Tooth No. 22 then would be force-erupted and extracted. This strategy would confine the bony defect to only the size of one tooth and change the character of the defect from having only one source of capillary in-growth to having three sources. Site No. 21 could be managed with mineralized freeze-dried bone allograft and a membrane. Implant site development requirements theoretically would be reduced, optimizing predictability and reducing surgical morbidity for the patient. Orthodontic tooth movement (eg, orthodontically directed distraction osteogenesis) would serve to rebuild the vertical height of the ridge and eliminate the need for an autogenous block graft. In addition, proper positioning of the cuspid via orthodontic tooth movement would create a more idealized occlusal scheme, provide a cuspid-protected occlusion, and minimize off-axis loading of the implant.35
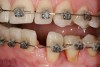
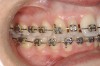
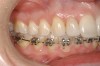
This treatment plan was elected with the premise to reevaluate treatment options during orthodontic tooth movement. Soft-tissue augmentation was initiated to correct the mucogingival defect in the area of teeth Nos. 21 and 22. A free gingival graft was performed to achieve an adequate band of attached and keratinized gingiva to resist potential stripping on the root surfaces of teeth Nos. 22 and 21 during orthodontic movement (Figure 8). Orthodontic movement then was initiated to site-develop position No. 23 for an implant (Figure 9 through Figure 11). Orthodontics also was used to idealize the patient’s occlusion and develop canine guidance to minimize occlusal load and maximize the redistribution of forces36-40 for protecting the future implant from deleterious forces. The space was opened around the peg lateral tooth No. 7, and the gingival levels were aligned for maxillary incisors. Bonding was performed to restore the length of teeth Nos. 8 through 10 and to bond the peg lateral to a normal tooth form, which allows the orthodontist to idealize the posterior occlusal scene and maintain canine guidance (Figure 12 and Figure 13).
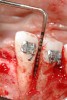
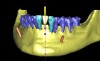
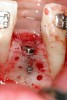
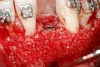
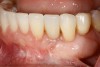
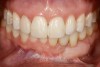
Following the first goal of orthodontia—moving tooth No. 22 to a proper functional and anatomical position—a periodontal reevaluation was performed, demonstrating an improvement in short- and long-term tooth prognosis. At this time, the decision was made to abort continual extrusion of teeth Nos. 21 and 22, although some attachment level discrepancy remained between Nos. 21 and 22 (Figure 14). Implant diagnostics ensued and included mounted study models and a diagnostic wax-up. A scanning appliance was created to demonstrate the desired prosthetic outcome requirements, and the patient was referred for computed tomography (CT) scans. CT scan assessment demonstrated successful orthodontically directed GBR to allow implant placement (Figure 15 and Figure 16). Only minor GBR therapy would be needed and could be accomplished simultaneously with implant installation. Guided implant placement occurred, using an open flap approach (Figure 17). During surgery, root dehiscences were noted on teeth Nos. 21, 22, 24, and 25, as well as the anticipated dehiscence following implant placement at No. 23 (Figure 18). A positioning reference (index) was secured after implant placement to facilitate a provisional prosthesis at stage II surgery. Cortical perforations then were placed adjacent to the implant to encourage angiogenesis (Figure 18), and mineralized freeze-dried bone allograft enhanced with platelet-derived growth factor was placed over the dehiscences for purposes of guided tissue regeneration and GBR (Figure 19). A highly resorbable collagen membrane was placed to stabilize the allograft. The flap was coronally repositioned, and primary-intention wound healing was achieved (Figure 20 and Figure 21). Following 4 months of stage I surgery, implant uncovery and immediate provisionalization were performed in conjunction with connective tissue grafting. Final orthodontic tooth movement then ensued, using the implant as anchorage to optimize end-tooth movements, interroot separation, and the cuspid-protected occlusal scheme. A final impression then was secured, and a zirconia abutment (Figure 22) with an all-ceramic restoration was fabricated for the prosthetic phase completion of No. 23 (Figure 23 through Figure 25).
This case illustrates how to manage dentoalveolar ridge destruction, using orthodontics, periodontics, and restorative dentistry to idealize the occlusal scheme, develop an ideal implant site with orthodontics, and enhance the dentoalveolar ridge dimensions surgically with bone graft materials. Finally, it demonstrates how to restore proper tooth contours through minimal restorative dentistry with bonding and a single-unit, implant-supported crown.
References
1. Fürhauser R, Florescu D, Benesch T, et al. Evaluation of soft tissue around single-tooth implant crowns: the pink esthetic score. Clin Oral Impl Res. 2005;16(6):639-644.
2. Gargiulo A, Wentz F, Orban B. Dimensions and relations of the dentogingval junction in humans. J Periodontol. 1961;32:261.
3. Sanavi F, Weisgold AS, Rose LF. Biologic width and its relation to periodontal biotypes. J Esthet Dent. 1998;10(3):157-163.
4. Becker W, Ochsenbein C, Tibbetts L, et al. Alveolar bone anatomic profiles as measured from dry skulls. Clinical ramifications. J Clin Periodontol. 1997;24(10):727-731.
5. Spear FM. Maintenance of the interdental papilla following anterior tooth removal. Pract Periodontics Aesthet Den. 1999;11(1):21-28.
6. Tarnow DP, Magner AW, Fletcher P. The effect of the distance from the contact point to the crest of bone on the presence or absence of the interproximal dental papilla. J Periodontol. 1992;63(12):995-996.
7. Kois JC. Altering gingival levels: the restorative connection part I: biologic variables. J Esthet Dent. 1994;6(1):3-7.
8. Vacek JS, Gher ME, Assad DA. The dimensions of the human dentogingival junction. Int J Periodontics Restorative Dent. 1994;14(2):154-165.
9. Berglundh T, Lindhe J. Dimension of the periimplant mucosa. Biologic width revisited. J Clin Periodontol. 1996;23(10):971-973.
10. Abrahamsson I, Berglundh T, Wennström J, et al. The peri-implant hard and soft tissues at different implant systems. A comparative study in the dog. Clin Oral Implants Res. 1996;7(3):212-219.
11. Hermann JS, Cochran DL, Nummikoski PV, et al. Crestal bone changes around titanium implants. A radiographic evaluation of unloaded nonsubmerged and submerged implants in the canine mandible. J Periodontol. 1997;68(11):1117-1130.
12. Cochran DL, Hermann JS, Schenk RK, et al. Biologic width around titanium implants. A histometric analysis of the implant-gingival junction around loaded and unloaded nonsubmerged implants in the canine mandible. J Periodontol. 1997;68(2):186-197.
13. Small PN, Tarnow DP. Gingival recession around implants: a 1-year longitudinal prospective study. Int J Oral Maxillofac Implants. 2000;15(4):527-532.
14. Hermann JS, Buser D, Schenk RK, et al. Crestal bone changes around titanium implants. A histometric evaluation of unloaded non-submerged and submerged implants in the canine mandible. J Periodontol. 2000;71(9):1412-1424.
15. Hermann JS, Schoolfield JD, Schenk RK, et al. Influence of the size of the microgap on crestal bone changes around titanium implants. A histometric evaluation of unloaded and loaded nonsubmerged implants in the canine mandible. J Periodontol. 2001;72(10):1372-1383.
16. Hermann JS, Buser D, Schenk RK, et al. Biologic width around one- and two-piece titanium implants. Clin Oral Implants Res. 2001;12(6):559-571.
17. Neale D, Chee WL. Development of implant soft tissue emergence profile: a technique. J Prosthet Dent. 1994;71(4):364-368.
18. Choquet V, Hermans M, Adriaenssens P, et al. Clinical and radiographic evaluation of the papilla level adjacent to single-tooth dental implants. A retrospective study in the maxillary anterior region. J Periodontol. 2001;72(10):1364-1371.
19. Grunder U, Gracis S, Capelli M. Influence of the 3-D bone-to-implant relationship on esthetics. Int J Periodontics Restorative Dent. 2005;25(2):113-119.
20. Panos P. Tooth ankylosis: orthodontic implications. Hel Orthod Rev. 2003;6:75-88.
21. Baccetti T. A controlled study of associated dental anomalies. Angle Orthod. 1998;68(3):267-274.
22. Fiorentino A, Vecchione P. Multiple congenitally missing teeth: treatment outcome with autologous transplantation and orthodontic space closure. Am J Orthod Dentofacial Orthop. 2007;132(5):693-703.
23. Schmitz JP, Hollinger JO. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res. 1986;205:299-308.
24. Wikesjo UME, Hanisch O, Sigurdsson T, et al. Application of rhBMP-2 to alveolar and periodontal defects. In: Lynch SE, Marx RE, Nevins M, et al, eds. Tissue Engineering: Applications in Maxillofacial Surgery and Periodontics. Hanover Park, IL: Quintessence Publishing; 2007:269-286.
25. Tinti C, Parma-Benfenati S. Clinical classification of bone defects concerning the placement of dental implants. Int J Periodontics Restorative Dent. 2003;23(2):147-155.
26. Wang HL, Boyapati L. “PASS” principles for predictable bone regeneration. Implant Dent. 2006;15(1):8-17.
27. Kokich VO Jr. Congenitally missing teeth: orthodontic management in the adolescent patient. Am J Orthod Dentofacial Orthop. 2002;121(6):594-595.
28. Gerstenfield LC, Culliname DM, Barnes GL, et al. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88(5):873-884.
29. Hicok KC, Hedrick MH. Stem cells and the art of mesenchymal maintenance. In: Bronner F, Farach-Carson MC, Mikos AG, eds. Engineering of Functional Skeletal Tissues. New York, NY: Springer; 2007.
30. Bouletreau PJ, Warren SM, Spector JA, et al. Hypoxia and VEGF up-regulate BMP2 mRNA and protein expression in microvascular endothelial cells: implications for fracture healing. Plast Reconstr Surg. 2002;109(7):2384-2397.
31. Fiorellini JP, Nevins ML. Localized ridge augmentation/preservation. A systematic review. Ann Periodontol. 2003;8(1):312-327.
32. Wagenberg BD, Eskow RN, Langer B. Orthodontic procedures that improve the periodontal prognosis. J Am Dent Assoc. 1980;100(3):370-373.
33. Matsumoto T, Kuroda R, Mifune Y, et al. Circulating endothelial/skeletal progenitor cells for bone regeneration and healing. Bone. 2008;43(3)434-439.
34. Tarnow DP, Cho SC, Wallace SS. The effect of inter-implant distance on the heights of the inter-implant bone crest. J Periodontol. 2000;71(4):546-549.
35. Sütpideler M, Eckert SE, Zobitz M, et al. Finite element analysis of effect of prosthesis height, angle of force application, and implant offset on supporting bone. Int J Oral Maxillofac Implants. 2004;19(6):819-825.
36. Manns A., Miralles R, Valdivia J, et al. Influence of variation in anteroposterior occlusal contacts on electromyographic activity. J Prosthet Dent. 1989;61(5):617-623.
37. MacDonald JW, Hannam AG. Relationship between occlusal contacts and jaw-closing muscle activity during tooth clenching: part I. J Prosthet Dent. 1984;52(5):718-728.
38. Manns A, Chan C, Miralles R. Influence of group function and canine guidance on electromyographic activity of elevator muscles. J Prosthet Dent. 1987;57(4):494-501.
39. Williamson EH, Lundquist DO. Anterior guidance: its effect on electromyographic activity of the temporal and masseter muscles. J Prosthet Dent. 1983;49(6):816-823.
40. Mansour RM, Reynik RJ. In vivo occlusal forces and moments: I. Forces measured in terminal hinge position and associated moments. J Dent Res. 1975;54(4):114-120.