You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Silver diamine fluoride (SDF) is a colorless alkaline topical fluoride solution containing fluoride ions and silver ions. Silver salts can provide a pronounced antimicrobial action, and they have a long history of use in medicine and dentistry.1 Meanwhile, the use of topical fluoride application has proven to be effective in dental caries prevention.2 Therefore, the combination of silver and fluoride has a hypothesized ability to cease the caries process and simultaneously prevent new caries.3
SDF has been used to manage dental caries in young children, arrest root caries in elderly patients, prevent pit and fissure caries and secondary caries, and desensitize teeth with hypersensitivity. Studies also suggest that SDF may be used to treat infected root canals and strengthen endodontically treated teeth.4 The promising results of both clinical and laboratory studies have suggested that SDF is more effective than fluoride varnish and may be a valuable caries-preventive intervention. A recent review concluded that SDF is a safe, effective, efficient, and equitable caries control agent that may meet the criteria of the World Health Organization (WHO) Millennium Development Goals and the US Institute of Medicine.3 The purpose of this article is to provide an overview of the clinical use of SDF in dental treatment. The current applications of SDF in dentistry are discussed.
Use of Silver Compounds in Dentistry
Silver compounds have been used for their medical properties for centuries.5 As early as the 1900s, silver compounds were popular regimens for treating tetanus and rheumatism before antibiotics were invented. When penicillin and other antibiotics emerged in the 1930s, research and clinical interest in silver were remarkably reduced. However, in the 1970s, interest in silver compounds reappeared due to the emergence of antibacterial resistance of some antibiotics.6 Today, silver is again favored as an antimicrobial agent due to its broad spectrum, low toxicity, and lack of cross-spectrum bacterial resistance.5,7
In dentistry, silver compounds have been used since as early as the 1840s, when silver nitrate was used to reduce the incidence of caries in the primary dentition.8 It was used as a caries-preventing agent for permanent molars, as well as a cavity sterilizing agent and dentin desensitizer.9,10 In the 1960s, silver was advocated to combine with fluoride as an anti-caries agent and presumably for a combined beneficial effect. One of the early publications in English that reported the topical application of silver fluoride to arrest dental caries was in Japan.11 Nishino found that the application of silver fluoride inhibits the lateral spread of dental caries.
Two different chemical forms are available for silver and fluoride combination: silver fluoride and SDF. Silver fluoride at 40% was used in western Australia to arrest dental caries in children.12 SDF contains ammonia and silver fluoride; the ammonia ions combine with silver ions to produce a complex ion called the diamine-silver ion, and this complex is more stable than silver fluoride. Thus, it is also called diamine silver fluoride. SDF is claimed to be more stable than silver fluoride, and it can be kept in constant concentration for a period of time.13 While silver fluoride is becoming less readily available in dentistry, SDF is commonly used in 38% solution for treatment of hypersensitivity and caries control. Some commercially available SDF products are listed in Table 1.
There are several advantages of using SDF in dental treatment. First, it showed an antimicrobial activity against mono-species, dual-species, and multi-species cariogenic biofilm.14-16 Silver ions are bactericidal metal cations that inhibit biofilm formation.17 Studies have indicated that silver interacts with sulfhydryl groups of proteins and DNA, thus altering hydrogen bonding and inhibiting respiratory processes, DNA unwinding, cell-wall synthesis, and cell division.18 At the macro level, these interactions affect bacterial killing and inhibit biofilm formation.17 Second, fluoride promotes caries lesion remineralization. Fluoride has been indicated to react with hydroxyapatite and generate calcium fluoride, which is a reservoir of fluoride, and facilitate further remineralization.19 An ex vivo study reported surface microhardness of the surface layer of the arrested caries after SDF applications was comparable with the unaffected sound dentin20 (Figure 1 and Figure 2). This is consistent with another study, in which a high remineralized zone was observed on the surface of arrested caries from exfoliated teeth with SDF treatment21 (Figure 3 and Figure 4). Third, its application procedures are simple and do not require injection or drilling, and the treatment does not involve expensive support infrastructure equipment such as piped water and electricity. The simplicity of the treatment is conducive to treating caries in apprehensive young children who may have intense dental fear, uncooperative patients with special needs, or elderly patients who have difficulty adapting to traditional dental care. It also allows trained workers to deliver the treatment to people who live in the area but who may not be able to easily access dental service.22 Patient compliance and satisfaction is often good when the patient is provided a clear explanation of the treatment outcome.23,24 Finally, the cost of SDF treatment is low and should be affordable in most communities.
The inherent disadvantage of SDF is that the caries lesions will be stained black after SDF application. SDF stops caries progression by forming a hard, blackened, impermeable layer on the tooth surface that is resistant to caries (Figure 5 through Figure 9). The authors’ clinical observations are that the darker the color, the more likely the caries arrested. Some patients may not be pleased with the esthetics of this treatment outcome; therefore, it is important to inform the patients and parents (for child patients) about this treatment outcome. Moreover, SDF can stain clothes and the skin of the body. Though it does not cause any pain or damage, an SDF stain on skin cannot be easily washed away. It takes around 7 days for it to disappear, and the stain on clothes is permanent.22
The SDF solution also has an unpleasant metallic taste. Furthermore, gingival and mucosal irritation may occur. In most cases, the affected tissue turns white and the change is transient.24 The white marks (burning) on the gingiva usually heal within 1 to 2 days. Other disadvantages include the solution’s sensitivity to light, and, hence, it must be kept in a dark/opaque container. The high fluoride concentration (44,800 ppm) of 38% SDF may cause dental fluorosis when it is applied in large doses on young children. In a study by the authors,16 the amount of SDF applied per application was approximately 27.5 µg/mm2. Although this amount of applied SDF is minute, precaution should be taken, and multiple and frequent applications on very small children should be avoided.22
To deal with the staining, some researchers suggested using potassium iodine, which reacts with the residual silver ion to eliminate the color stain effect.1,25 However, a clinical trial found that staining of the arrested carious lesions could not be removed through this method.26 Ammonium hexafluorosilicate was proposed to be used instead of SDF, but acid resistance of the teeth after application was inferior to those treated with SDF.27 A study used nano silver fluoride and found that the treated carious lesion had no significant staining.28,29 More laboratory and clinical studies should be carried out before it is recommended for clinical use.
Use of SDF for Dental Treatment
Caries Control
Although SDF is cleared by the US Food and Drug Administration as a fluoride for treatment of tooth hypersensitivity, it is often used for caries control and management. It can be directly applied on a sound tooth surface for prevention30 or on a carious lesion for arrestment.23,31 SDF does not stain sound enamel. The teeth to be treated with SDF can be dried and isolated with cotton rolls. The authors’ unpublished results found very low incidence of gingival irritation (<6%), which was spontaneously resolved within 2 days. Instruction by the manufacturer of an SDF product suggests protecting the gingival tissue of the affected tooth with petroleum jelly/rubber before SDF is applied via a disposable microbrush. It seems logical that caries should be largely removed before SDF application. The authors’ clinical trial found no difference in the caries arrest rate with or without caries removal prior to 38% SDF application.23 The use of SDF without caries removal in community-based programs has the advantage of being simple and easily obtaining good cooperation from young children or the elderly. It is noteworthy that it takes longer for the carious tissue to be arrested if it is not removed.32
There is no consensus on the frequency of application, and 38% SDF has been used annually or biannually on clinical trials in children23,31,33,34 and in elderly.30,35 Yee and his co-worker found one-off application of 12% SDF was ineffective in arresting caries in children.31 The present authors applied 38% SDF weekly for 3 weeks to speed up the process of caries arrest and for treatment of rampant caries.36 One of the present authors’ case reports demonstrated that three weekly applications of 38% SDF can arrest rampant caries and relieve pain from hypersensitivity on a teenager. The SDF-treated caries were found arrested, and they turned coal black in appearance (Figure 9).
Management of Hypersensitivity
Dentin hypersensitivity may occur on an exposed dentin surface.37 It is characterized by varying degrees of pain that can be initiated by thermal, evaporative, tactile, chemical, or osmotic stimuli. SDF was used to treat dentin hypersensitivity.38,39 The clinical procedure is similar to treating caries. The hypersensitive teeth can be isolated with cotton rolls. The area to be treated is gently dried, and SDF is applied with a disposable microbrush. Knight and co-workers suggest applying potassium iodide immediately after SDF application. In their study, they found that potassium iodide could further reduce dentin permeability when it was applied after topical fluoride treatment.1 Studies found that both SDF and SDF plus potassium iodide are effective and safe in desensitizing teeth 1 week after treatment.38,39
Disinfection of Root Canal in Endodontic Treatment
The elimination of microorganisms of root canal in endodontic treatment is fundamental for a successful treatment. Several antibacterial agents were used for root canal disinfection but resistance of the Enterococcus faecalis was reported.40 SDF at 3.8% (Saforide 3.8%, Toyo Seiyaku Kasei Co. Ltd., https://www.teikoku.co.jp/english/index_eng.html) is available for root canal disinfection in endodontic treatment. This represents a 1:10 dilution of the 38% SDF solution and is recommended by the manufacturer to be applied three times at 24-hour intervals. A laboratory study showed that 3.8% SDF exhibited 100% reduction of E. faecalis after 60-minute exposure.41 The SDF stained the root canal, and the application time of SDF was associated with the percentage of precipitates on pulpal dentin. SDF is also suggested to be used as an antimicrobial root canal irrigant or inter-appointment dressing.41,42 Clinical data is needed to support the laboratory findings.
Other Uses of SDF for Dental Treatment
Apart from caries control, management of tooth hypersensitivity, and endodontic treatment, SDF and laser irradiation can be used to strengthen dentin for caries prevention. Studies have shown that Er:YAG (2,980 nm) and CO2 (10,600 nm) laser irradiation could increase the fluoride uptake of SDF on dentin.43,44 Zhang mentioned that several mechanisms may contribute to the combined effect of fluoride and laser irradiation, including: alteration in the composition of recrystallized hydroxyapatite, resulting in decreased solubility; enhanced uptake of fluoride to form fluoride apatite; and micropores created in the mineral structure, providing sites for precipitation of calcium, phosphate, and fluoride.45 This can be one of the main reasons why 38% SDF application followed by laser irradiation can reduce the risk of tooth fracture of endodontically treated teeth.46 Although laboratory studies demonstrated that this adjunctive application of laser irradiation and SDF can strengthen dentin, clinical study is lacking.
Safety
Silver diamine fluoride (SDF) has been in use in many countries, including Australia and China, for many years to arrest dental caries.22 In Japan, it has been accepted for more than 50 years by the Central Pharmaceutical Council of the Ministry of Health and Welfare for dental treatment as a therapeutic agent. Like the use of silver amalgam in dentistry, SDF has a long-proven success, and there are no significant complications reported in the literature. Two studies investigating oral tissue response to 38% SDF found transient gingival irritation, but there was no severe pulpal damage or severe reaction reported.11,47 Llodra and co-workers found that gingival irritation was resolved within 2 days of no treatment.24 A clinical study measured the gingival erythema and found that no patients developed severe erythema after SDF application.38 Our clinical trial found no severe reaction of SDF application on young preschool children.23
SDF at 38% contains a high concentration of fluoride (44,800 ppm), which may raise concern for its risk when inducing dental fluorosis on young children. Dental fluorosis is caused by excess fluoride ingestion results. The severity of the fluorosis is related to the concentration of fluoride in the plasma.48 Although there is no study performed on children, a study measured the serum concentration for fluoride and silver in adults after topical SDF application.49 The results showed that fluoride exposure was below the US Environmental Protection Agency (EPA) oral reference dose. Moreover, the researchers concluded that occasional use of SDF is well below the concentrations associated with toxicity.
Conclusion
Studies and clinical cases have found that SDF has a broad application in dentistry. SDF treatment is an efficient, simple, quick, and safe method of dental treatment. It has shown to be an effective agent in preventing new caries and arresting existing caries. It can also be employed to treat dentin hypersensitivity. In addition, it can be used to disinfect root canal(s) in endodontics treatment. Its combined application with laser irradiation could increase the fluoride uptake of teeth and the prevention of tooth fracture after root canal treatment. SDF at 38% contains a high concentration of silver and fluoride ions. Yet the current literature has no evidence of its use to cause dental fluorosis, gingival irritation, or high toxicity. The black staining of an arrested carious lesion can be an esthetic concern of SDF treatment; thus, the patients must be informed about this treatment outcome.
Disclosure
The authors had no disclosures to report.
About the Authors
May L. Mei, BDS, MDS
PhD Faculty of Dentistry
University of Hong Kong
Edward Chin-Man Lo, BDS, MDS, Ph
Faculty of Dentistry
University of Hong Kong
Chun-Hung Chu, BDS, MDS, Ph
Faculty of Dentistry
University of Hong Kong
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Knight GM, McIntyre JM, Craig GG, et al. An in vitro model to measure the effect of a silver fluoride and potassium iodide treatment on the permeability of demineralized dentine to Streptococcus mutans. Aust Dent J. 2005;50(4):242-245.
2. Chu CH, Mei ML, Lo EC. Use of fluorides in dental caries management. Gen Dent. 2010;58(1):37-43; quiz 44-45, 79-80.
3. Rosenblatt A, Stamford TC, Niederman R. Silver diamine fluoride: a caries “silver-fluoride bullet.” J Dent Res. 2009;88(2):116-125.
4. Yokoyama K, Kimura Y, Matsumoto K, et al. Preventive effect of tooth fracture by pulsed Nd:YAG laser irradiation with diamine silver fluoride solution. J Clin Laser Med Surg. 2001;19(6):315-318.
5. Peng JJ, Botelho MG, Matinlinna JP. Silver compounds used in dentistry for caries management: a review. J Dent. 2012;40(7):531-541.
6. Spadaro JA, Webster DA, Becker RO. Silver polymethyl methacrylate antibacterial bone cement. Clin Orthop Relat Res. 1979;(143):266-270.
7. Duffin S. Back to the future: the medical management of caries introduction. J Calif Dent Assoc. 2012;40(11):852-858.
8. Stebbins EA. What value has argenti nitras as a therapeutic agent in dentistry? Int Dent J. 1891;12:661-670.
9. Everett FG, Hall WB, Phatak NM. Treatment of hypersensitive dentin. J Oral Ther Pharmacol. 1966;2(4):300-310.
10. James PMC, Parfitt GJ. A clinical note on the use of silver nitrate in the prevention of fissure caries in newly erupted first permanant molars. Br Dent J. 1954;96:35.
11. Nishino M. [Studies on the topical application of ammoniacal silver fluoride for the arrest of dental caries]. Osaka Daigaku Shigaku Zasshi. 1969;14(1):1-14.
12. Gotjamanos T. Pulp response in primary teeth with deep residual caries treated with silver fluoride and glass ionomer cement (‘atraumatic’ technique) Aust Dent J. 1996;41(5):328-334.
13. Mei ML, Chu CH, Lo EC, Samaranayake LP. Fluoride and silver concentrations of silver diammine fluoride solutions for dental use. Int J Paediatr Dent. 2013;23(4):279-285.
14. Mei ML, Chu CH, Lo EC, Samaranayake LP. Preventing root caries development under oral biofilm challenge in an artificial mouth. Med Oral Patol Oral Cir Bucal. 2013;18(4):e557-e563.
15. Mei ML, Chu CH, Low KH, et al. Caries arresting effect of silver diamine fluoride on dentine carious lesion with S. mutans and L. acidophilus dual-species cariogenic biofilm. Med Oral Patol Oral Cir Bucal. 2013;18(6):e824-e831.
16. Chu CH, Mei L, Seneviratne CJ, Lo EC. Effects of silver diamine fluoride on dentine carious lesions induced by Streptococcus mutans and Actinomyces naeslundii biofilms. Int J Paediatr Dent. 2012;22(1):2-10.
17. Wu MY, Suryanarayanan K, van Ooij WJ, Oerther DB. Using microbial genomics to evaluate the effectiveness of silver to prevent biofilm formation. Water Sci Technol. 2007;55(8-9):413-419.
18. Oppermann RV, Johansen JR. Effect of fluoride and non-fluoride salts of copper, silver and tin on the acidogenicity of dental plaque in vivo. Scand J Dent Res. 1980;88(6):476-480.
19. ten Cate JM, Damen JJ, Buijs MJ. Inhibition of dentin demineralization by fluoride in vitro. Caries Res. 1998;32(2):141-147.
20. Chu CH, Lo EC. Microhardness of dentine in primary teeth after topical fluoride applications. J Dent. 2008;36(6):387-391.
21. Mei ML, Ito L, Cao Y, et al. An ex vivo study of arrested primary teeth caries with silver diamine fluoride therapy. J Dent. 2014;42(4):395-402.
22. Chu CH, Lo EC. Promoting caries arrest in children with silver diamine fluoride: a review. Oral Health Prev Dent. 2008;6(4):315-321.
23. Chu CH, Lo EC, Lin HC. Effectiveness of silver diamine fluoride and sodium fluoride varnish in arresting dentin caries in Chinese pre-school children. J Dent Res. 2002;81(11):767-770.
24. Llodra JC, Rodriguez A, Ferrer B, et al. Efficacy of silver diamine fluoride for caries reduction in primary teeth and first permanent molars of schoolchildren: 36-month clinical trial. J Dent Res. 2005;84(8):721-724.
25. Hamama HH, Yiu CK, Burrow MF. Effect of silver diamine fluoride and potassium iodide on residual bacteria in dentinal tubules. Aust Dent J. 2015;60(1):80-87.
26. Li R, Lo CM, Chu CH, Liu BY. Arresting root caries through fluoride applications – 2-year results [abstract]. J Dent Res. 2015;94(spec iss A). Abstract 2886.
27. Kawasaki A, Suge T, Ishikawa K, et al. Ammonium hexafluorosilicate increased acid resistance of bovine enamel and dentine. J Mater Sci Mater Med. 2005;16(5):461-466.
28. Targino AG, Flores MA, dos Santos Junior VE, et al. An innovative approach to treating dental decay in children. A new anti-caries agent. J Mater Sci Mater Med. 2014;25(8):2041-2047.
29. Santos VE Jr, Vasconcelos Filho A, Targino AG, et al. A new “silver-bullet” to treat caries in children—nano silver fluoride: a randomised clinical trial. J Dent. 2014;42(8):945-951.
30. Tan HP, Lo EC, Dyson JE, et al. A randomized trial on root caries prevention in elders. J Dent Res. 2010;89(10):1086-1090.
31. Yee R, Holmgren C, Mulder J, et al. Efficacy of silver diamine fluoride for Arresting Caries Treatment. J Dent Res. 2009;88(7):644-647.
32. Wong MC, Lam KF, Lo EC. Analysis of multilevel grouped survival data with time-varying regression coefficients. Stat Med. 2011;30(3):250-259.
33. Duangthip D, Chu CH, Lo CM. A randomized clinical trial on arresting dentine caries in preschool children by topical fluorides-18 month results. J Dent. 2015; doi: 10.1016/j.jdent.2015.05.006. [Epub ahead of print]
34. Zhi QH, Lo EC, Lin HC. Randomized clinical trial on effectiveness of silver diamine fluoride and glass ionomer in arresting dentine caries in preschool children. J Dent. 2012;40(11):962-967.
35. Zhang W, McGrath C, Lo EC, Li JY. Silver diamine fluoride and education to prevent and arrest root caries among community-dwelling elders. Caries Res. 2013;47(4):284-290.
36. Chu CH, Lee AH, Zheng L, et al. Arresting rampant dental caries with silver diamine fluoride in a young teenager suffering from chronic oral graft versus host disease post-bone marrow transplantation: a case report. BMC Res Notes. 2014;7:3.
37. Chu CH, Lam A, Lo EC. Dentin hypersensitivity and its management. Gen Dent. 2011;59(2):115-122; quiz 123-124.
38. Castillo JL, Rivera S, Aparicio T, et al. The short-term effects of diammine silver fluoride on tooth sensitivity: a randomized controlled trial. J Dent Res. 2011;90(2):203-208.
39. Craig GG, Knight GM, McIntyre JM. Clinical evaluation of diamine silver fluoride/potassium iodide as a dentine desensitizing agent. A pilot study. Aust Dent J. 2012;57(3):308-311.
40. Law A, Messer H. An evidence-based analysis of the antibacterial effectiveness of intracanal medicaments. J Endod. 2004;30(10):689-694.
41. Hiraishi N, Yiu CK, King NM, et al. Antimicrobial efficacy of 3.8% silver diamine fluoride and its effect on root dentin. J Endod. 2010;36(6):1026-1029.
42. Mathew VB, Madhusudhana K, Sivakumar N, et al. Anti-microbial efficiency of silver diamine fluoride as an endodontic medicament - an ex vivo study. Contemp Clin Dent. 2012;3(3):262-264.
43. Mei ML, Ito L, Chu CH, et al. Prevention of dentine caries using silver diamine fluoride application followed by Er:YAG laser irradiation: an in vitro study. Lasers Med Sci. 2014;29(6):1785-1791.
44. Mei ML, Ito L, Zhang CF, et al. Effect of laser irradiation on the fluoride uptake of silver diamine fluoride treated dentine. Lasers Med Sci. 2015;30(3):985-991.
45. Zhang C, Kimura Y, Matsumoto K. The effects of pulsed Nd:YAG laser irradiation with fluoride on root surface. J Clin Laser Med Surg. 1996;14(6):399-403.
46. Yokoyama K, Matsumoto K, Murase J. Permeability of the root canal wall and occlusion of dentinal tubules by Ag(NH3)2F: a comparison of combined use with pulsed Nd:YAG laser or iontophoresis. J Clin Laser Med Surg. 2000;18(1):9-14.
47. Okuyama T. [On the penetration of diammine silver fluoride into the carious dentin of deciduous teeth (author’s transl)]. Shigaku. 1974;61(6):1048-1071.
48. Denbesten P, Li W. Chronic fluoride toxicity: dental fluorosis. Monogr Oral Sci. 2011;22:81-96.
49. Vasquez E, Zegarra G, Chirinos E, et al. Short term serum pharmacokinetics of diammine silver fluoride after oral application. BMC Oral Health. 2012;12:60.
![Fig 1. Ground section of a primary incisor with arrested caries lesion after SDF treatment. Fig 1: Arrested caries that had SDF treatment. Fig 2: Microhardness of dentin (in median Knoop hardness number) in soft and SDF-arrested caries according to the distance from the lesion surface. (images from Chu and Lo, 200820 [reprinted with approval])](/media/thumbnail/20662)
![Fig 1 and Fig 2. Ground section of a primary incisor with arrested caries lesion after SDF treatment. Fig 1: Arrested caries that had SDF treatment. Fig 2: Microhardness of dentin (in median Knoop hardness number) in soft and SDF-arrested caries according to the distance from the lesion surface. (images from Chu and Lo, 200820 [reprinted with approval])](/media/thumbnail/20663)
![Fig 3. Scanning electron microscope (SEM) images of the dentin carious lesions. Fig 3: Surface morphology of arrested carious lesion. Fig 4: Surface morphology of active carious lesion. (images from Mei, et al, 201421 [reprinted with approval])](/media/thumbnail/20664)
![Fig 4. Scanning electron microscope (SEM) images of the dentin carious lesions. Fig 3: Surface morphology of arrested carious lesion. Fig 4: Surface morphology of active carious lesion. (images from Mei, et al, 201421 [reprinted with approval])](/media/thumbnail/20665)
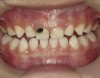
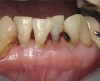
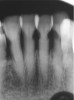
![Fig 8. Use of 38% SDF to arrest rampant caries in a young teenager. Fig 8: Pre-treatment intraoral frontal view of rampant caries. Fig 9: Frontal view of arrested caries after consecutive application of SDF for 3 weeks. (images from Chu, et al, 201436 [reprinted with approval])](/media/thumbnail/20669)
![Fig 9. Use of 38% SDF to arrest rampant caries in a young teenager. Fig 8: Pre-treatment intraoral frontal view of rampant caries. Fig 9: Frontal view of arrested caries after consecutive application of SDF for 3 weeks. (images from Chu, et al, 201436 [reprinted with approval])](/media/thumbnail/20670)
