You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Advances in digital dental technology are directly related to progress in computer science and transistor and microchip technology. The digital revolution we are experiencing today can be traced back to 1947, when the first transistor was invented by Bell Laboratory engineers and Nobel Prize winners John Bardeen, Walter Brattain, and William Shockley. Initial transistors were slow, bulky, and very few were included in integrated circuits or microchips. Technology has created modern transistors that are extremely small (as small as 1 atom thick and 10 atoms across), extremely fast (GHz speed), and densely packed in microchips. For example, the Core i-series processor released in 2010 contains 1.17 billion transistors compared to only 2,300 transistors in the early 1970s.1
Newer microchips continue to develop quickly. According to Moore’s law, every 12 to 24 months a new microchip is expected and each new microchip contains roughly twice as much capacity, processor power, and disk drive storage as its predecessor.1 Therefore, it is not surprising that dentistry is experiencing a recent explosion in digital dental technology, software, scanning, and manufacturing capabilities. Not only is digital radiography routine practice in dental clinics, but virtual diagnosis and virtual treatment planning are also becoming mainstream. Digital impressions and the virtual patient are a reality.
The first attempt to create optical impressions of oral structures dates back to 1973, when Francois Duret, then a PhD student at the Claude-Bernard University in Lyon, France, conceived the idea of using a laser to obtain optical impressions that could be used for diagnosis, treatment planning, and the fabrication and machining of restorations.2 In 1983, Drs. Werner Mörmann and Marco Brandestini developed the first intraoral scanner for restorative dentistry that was theoretically capable of achieving an accuracy of 50 to 100 μm.3,4 This scanner relied on the principles of triangulation to produce instantaneous three-dimensional (3D) images of a tooth, and was used as part of a comprehensive system to acquire optical impressions and design and mill restorations. This computer-assisted CERamic REConstruction or Chairside Economical Restoration of Esthetic Ceramics (CEREC) system was first used to fabricate chairside inlays but was further developed through the years to create 3D images of tooth structures and to design and fabricate single full-coverage restorations and fixed dental prostheses.3,5 The CEREC system has since been upgraded several times, culminating recently with the introduction of the CEREC Omnicam system (Sirona Dental, www.sironausa.com).
The CEREC system receives considerable attention because it was the first to be introduced on the market and remained the sole optical solution for decades before the introduction of newer systems. Currently, there are several systems for acquiring intraoral optical impressions and CAD/CAM restorations on the market and most of them rely on the triangulation principle to acquire optical impressions. Examples of such systems include the TRIOS® (3Shape, www.3shape.com), the iTero® Element™ (Align Technology, www.aligntech.com), and the 3M™ True Definition Scanner (3M ESPE, www.3mespe.com).6 For a detailed discussion of the different digital systems available on the market, the reader is referred to materials included in the textbook Clinical Applications of Digital Dental Technology.7
Advantages of Current Digital Systems
The ability to accurately and non-invasively acquire a digital replica of dentoalveolar structures is common to the systems that are currently available. This is an immediately apparent advantage over conventional impressions. Optical impressions are easily adapted in the clinical workflow and require a shallow learning curve. Therefore, they can be more efficient, more comfortable for patients, and improve the profitability of dental practice. They produce an optical replica of the teeth, rather than a negative replica, and therefore can be evaluated easily in 3D and real time for the presence of defects and completeness. They do not require physical space for storage or disinfection.8 Impression materials are also not used, thus mitigating the risk of dimensional changes and distortion.8 Research has shown that the accuracy of optical impressions is comparable to, if not better than, conventional impressions.9-11
Perhaps the major advantage of these systems is their ability to facilitate and enhance diagnosis and treatment planning and, as a result, refine treatment outcomes. Optical impressions can be obtained, visualized, and virtually articulated immediately. Tooth position, contours, existing restorations, defects, edentulous spaces, occlusal contacts at maximum intercuspation, and available restorative space can therefore be evaluated using high-resolution 3D images. In newer systems (eg, TRIOS; CEREC Omnicam), optical impressions are acquired in color, replicating the natural appearance of teeth and gingiva. This allows the clinician to distinguish between the different types of restorative materials (eg, metals, porcelain, enamel, composite), to recognize inflamed and bleeding areas, to identify areas with plaque and calculus accumulation, and to evaluate color transitions on teeth.
Optical impressions can also be used as a powerful tool to demonstrate and discuss clinical findings and diagnosis with the patients. Enhanced 3D images of defective restorations, tooth wear, tooth super eruption and angulation, and tooth defects can be magnified and instantaneously reviewed with the patient without the need for physical models (Figure 1). This enables the clinician to educate the patient, increase patient acceptance,12 and enhance patient cooperation. Digital files of optical impressions are typically saved and stored as surface tessellation files (STL), and if desired, optical impressions can also be transformed to physical casts using additive or subtractive manufacturing technologies.13
Preparing for Optical Impressions
As with conventional impressions, optical impressions are sensitive to the presence of blood and saliva, and the teeth should be reasonably dry and clean before making an impression. Optical impressions are also sensitive to lighting conditions and, depending on the system, they can be affected by reflective surfaces. They are acquired using optical wands and, depending on the wand size and dimensions, access to posterior areas in the mouth (eg, distal of second molars) may be challenging. The wand, like a conventional tray, may also trigger a gag reflex.8
In a digital workflow, optical impressions are obtained following a comprehensive patient examination that includes a review of medical and dental history, a thorough intraoral and extraoral examination, and a clear understanding of the patient’s expectations. This diagnostic information can then be used to formulate a comprehensive treatment plan that addresses the patient’s chief complaint.14 Recent technological advances in software capability allow the dentist to perform digital “mock-ups” in lieu of a physical wax-up on diagnostic casts. Design, contours, position, dimensions, proximal contacts, emergence profile, and occlusion of restorations can be planned virtually and visualized in 3D, allowing for the fabrication of a provisional restoration before tooth preparation. However, a limitation of existing digital technology is the minimal emphasis of eccentric jaw movement and occlusal determinants on restoration design. The relationship of the maxilla to a reference plane cannot be easily established.15 Therefore, the inclination of the occlusal plane when multiple anterior teeth are being restored is difficult to determine. Patient-specific parameters of condylar inclination, lateral translation, and other determinants of occlusion are also difficult to program. The use of these systems for treatment planning of extensive rehabilitation provides its own challenges. Impressions of soft tissues prove problematic as well, and limit the use of intraoral optical scanners for capturing impressions of edentulous ridges. These caveats aside, the ability to visualize planned treatment in 3D without the need for physical casts and a wax-up enhances efficiency and patient understanding, which may result in improved acceptance of treatment plans and improved outcomes.
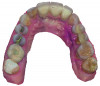
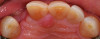
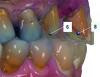
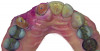
Based on the virtual plan, the treatment is carried out. An example of virtually planned and executed treatment is shown in Figure 2 through Figure 7. The patient presented with a missing maxillary right lateral incisor (Figure 2). Based on the patient’s desires, expectations, and comprehensive examination, a lithium disilicate fixed dental prosthesis (FDP) was planned to restore the missing lateral incisor. A virtual mock-up of the desired treatment plan was performed to delineate tooth length, width, and emergence profile (Figure 3). The treatment plan was then executed by preparing the abutment teeth to receive the FDP (Figure 4). An optical impression was made of the preparations and opposing teeth, and the resulting scans were virtually articulated (Figure 5). The optical impression was used to evaluate all aspects of the preparation, including finish line width and finish, path of insertion, and occlusal and axial reduction. It was also examined for the presence of undercuts, which were delineated by a red color (Figure 6). Errors in preparation design can be easily corrected and a new optical impression of the modified areas can be made without having the patient return for another visit. Once completed, the digital files can be sent to the laboratory for restoration design and manufacture, or used to design and fabricate the restoration using an in-house milling machine.14 An example of a completed restoration is shown in Figure 7.
CBCT and Scanning Protocol
The use of digital dentistry in diagnosis and treatment planning is not new (see American College of Prosthodontists Position Paper on digital dentistry16). For decades, dentists used specialized software to visualize 3D computed tomography (CT) scans of orofacial structures to evaluate skeletal growth; joint pathology; bone architecture; dimensions; vital structures including blood vessels and nerves, sinuses, and impacted teeth; and the presence of neoplasms. Driven by advances in imaging technology and computer power, digital technology is now used extensively in diagnosis and treatment planning of endosseous implants and in maxillofacial surgery.17 A major technological advance was the introduction of cone-beam computed tomography (CBCT).18 Compared to conventional CT scans, CBCT scans offer reduced exposure to x-rays at a reduced cost. Indeed, the total radiation caused by a CBCT scanner is 20% less than that of a helical CT and is approximately equal to exposure from a complete conventional radiographic survey using periapical films.19
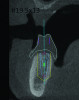
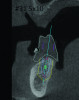
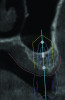
3D radiographic files (CT, CBCT) are stored digitally using a standardized digital imaging and communication in medicine (DICOM) format. In combination with a radiographic guide fabricated from a diagnostic wax-up, CBCT can be used to plan implant location and angulation based on prosthetically driven parameters and local bone/tissue constraints (Figure 8 through Figure 11). Currently, there are two different protocols to incorporate the radiographic guide in 3D scans and to perform computer guided surgery. The first is the double scan protocol, in which the patient wearing the radiographic guide and the radiographic guide alone are scanned separately. Fiduciary markers incorporated in the radiographic guide allow for matching of the two scans. This protocol allows for digitization of the surgical guide with great accuracy, irrespective of errors introduced by patient scans.18 Specialized software can be used to create a surgical guide (Figure 12).
The second protocol is the single scan protocol. In this protocol, the patient is scanned once while wearing the radiographic guide. Imaging files can be imported to the implant planning software without the need for further processing. As with the double scan protocol, the location and position of implants can be planned and a surgical guide can be fabricated based on the virtual plan.
3D radiographic scans obtained using the single scan protocol can be combined with digital mock-ups performed on intraoral optical scans or scans of casts,20 using the existing natural teeth as fiduciary markers. Different masks for bone, teeth, gingiva, and implants can be designated and processed (Figure 13 and Figure 14). This technology allows for greater accuracy, since implant position will be determined based on natural teeth (fiduciary markers) that are likely to be more stable and accurate than fiduciary markers placed on a radiographic guide.
Regardless of the scanning protocol used, digital advances in 3D imaging, optical scanning, and processing software endow the dentist with unprecedented tools to visualize and examine orofacial structures in 3D. This translates into better diagnosis and treatment planning. By evaluating soft tissue dimensions and contours, bone dimensions and quality, and the location of nerves and vital structures, the dentist can virtually plan implant placement surgery. Indeed, common to all scanning protocols discussed above, the resulting surgical guide reproduces the virtual implant position accurately and without allowing for intraoperative modification of implant position. The dentist can also execute this virtual plan with great accuracy, which is likely to ensure a favorable treatment outcome.
Final Thoughts
Intraoral optical scanners are being constantly modified to acquire faster, extremely accurate scans using miniaturized equipment. Many resources are available for additional learning on digital technology, including a digital dentistry symposium administered yearly by the American College of Prosthodontists (www.prosthodontics.org/continuing-education/digital-dentistry-symposium). In combination with continued development of 3D imaging technology and processing software, dentists are living in the golden age of digital technology. These advances are only likely to improve dentists’ ability to diagnose, treatment plan, and enhance patients’ comfort and quality of life. Thus, it is imperative that newer digital technologies are applied and embraced in dental offices.
Author Information
Radi Masri, DDS, MS, PhD, is associate editor of the Journal of Prosthodontics as well as a diplomate of the American Board of Prosthodontics, a fellow of the American College of Prosthodontists, a fellow of the American Academy of Fixed Prosthodontics, and a fellow of the Academy of Prosthodontics. An expert in fixed, removable, and implant dentistry, Masri is an associate professor with tenure at the University of Maryland School of Dentistry and School of Medicine. He lectures nationally and internationally and serves as an external examiner for international dental schools in the field of prosthodontics. He is also a director of the American Board of Prosthodontics.
Carl F. Driscoll, DMD, is a professor at the University of Maryland School of Dentistry, where he is also director of the prosthodontics residency. He previously held the same position with the United States Army at Walter Reed Army Medical Center in Washington, DC, from 1994 to 1997. Driscoll retired from the U.S. Army in 1997 with the rank of Colonel. Driscoll is currently president of the American College of Prosthodontists (ACP). He served as the chair of the ACP Board Preparation Course for 5 years and was a member of the American Dental Association’s (ADA’s) Commission on Dental Accreditation Review Board.
Disclosure
Drs. Masri and Driscoll receive royalties for their textbook Clinical Applications of Digital Dental Technology.
References
1. Mueller S. Upgrading and Repairing PCs. Cranbury, NJ: Pearson Education, Inc.; 2012.
2. Duret F. The Optical Impression [thesis]. Lyon, France: Universite Claude-Bernard; 1973:285.
3. Mörmann WH. The evolution of the CEREC system. J Am Dent Assoc. 2006;137 Suppl:7S-13S.
4. Hehn S. The evolution of a chairside CAD/CAM system for dental restorations. Compend Contin Educ Dent. 2001;22(6 Suppl):4-6.
5. Poticny DJ, Klim J. CAD/CAM in-office technology: innovations after 25 years for predictable, esthetic outcomes. J Am Dent Assoc. 2010;141 Suppl 2:5S-9S.
6. Zimmermann M, Mehl A, Mormann WH, Reich S. Intraoral scanning systems - a current overview. Int J Comput Dent. 2015;18(2):101-129.
7. Masri R, Driscoll CF. Clinical Applications of Digital Dental Technology. Hoboken, NJ: Wiley-Blackwell; 2015.
8. Gary GD, Bloom IT, Patzelt SBM. Digital impressions. In: Masri R, Driscoll CF, eds. Clinical Applications of Digital Dental Technology. Hoboken, NJ: Wiley-Blackwell; 2015:27-41.
9. Patzelt SB, Bishti S, Stampf S, Att W. Accuracy of computer-aided design/computer-aided manufacturing-generated dental casts based on intraoral scanner data. J Am Dent Assoc. 2014;145(11):1133-1140.
10. Ender A, Mehl A. Full arch scans: conventional versus digital impressions--an in-vitro study. Int J Comput Dent. 2011;14(1):11-21.
11. Cho SH, Schaefer O, Thompson GA, Guentsch A. Comparison of accuracy and reproducibility of casts made by digital and conventional methods. J Prosthet Dent. 2015;113(4):310-315.
12. Grünheid T, McCarthy SD, Larson BE. Clinical use of a direct chairside oral scanner: an assessment of accuracy, time, and patient acceptance. Am J Orthod Dentofacial Orthop. 2014;146(5):673-682.
13. Grant GT. Direct digital manufacturing. In: Masri R, Driscoll CF, eds. Clinical Applications of Digital Dental Technology. Hoboken, NJ: Wiley-Blackwell;2015:41-57.
14. Fasbinder DJ, Neiva GF. Digital application in operative dentistry. In: Masri R, Driscoll CF, eds. Clinical Applications of Digital Dental Technology. Hoboken, NJ: Wiley Blackwell; 2015:57-75.
15. Solaberrieta E, Otegi JR, Minguez R, Etxaniz O. Improved digital transfer of the maxillary cast to a virtual articulator. J Prosthet Dent. 2014;112(4):921-924.
16. Campbell SD. Digital dentistry. American College of Prosthodontists position statement. http://www.gotoapro.org/assets/1/7/3._Digital_Dentistry_-_Affirmed.pdf. Accessed August 31, 2015.
17. Greenberg AM. Digital technologies for dental implant treatment planning and guided surgery. Oral Maxillofac Surg Clin North Am. 2015;27(2):319-340.
18. Weber HP, Cano J, Bonino F. Digital implant surgery. In: Masri R, Driscoll CF, eds. Clinical Applications of Digital Dental Technology. Hoboken, NJ: Wiley-Blackwell; 2015:139-167.
19. Mah JK, Danforth RA, Bumann A, Hatcher D. Radiation absorbed in maxillofacial imaging with a new dental computed tomography device. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003; 96(4):508-513.
20. Lanis A, Álvarez Del Canto O. The combination of digital surface scanners and cone beam computed tomography technology for guided implant surgery using 3Shape implant studio software: a case history report. Int J Prosthodont. 2015;28(2):169-178.