You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
In November 2008, the Food and Drug Administration (FDA) approved tapentadol HCl (Nucynta™, Ortho-McNeil-Janssen Pharmaceuticals, www.ortho-mcneil.com) for the treatment of acute pain.1 Tapentadol is a centrally acting narcotic (opioid) analgesic with a dual mechanism of action.2 Like classic narcotics such as morphine and hydrocodone, tapentadol activates μ-opioid receptors. In addition, similar to tricyclic antidepressants, tapentadol blocks the neuronal reuptake of norepinephrine, which, in turn, increases synaptic concentrations of this neurotransmitter. Tapentadol is available in 50-mg, 75-mg, and 100-mg immediate-release tablets and is indicated for moderate-to-severe acute pain.
Narcotic Analgesic Use in Dentistry
Narcotic analgesics in fixed-dose combinations with acetaminophen or ibuprofen are effective in the treatment of postsurgical dental pain.3-8 Often, these types of drugs are referred to as narcotic combination drugs and take advantage of a strategy termed cross-firing in that they block pain production by two different mechanisms—prostaglandin inhibition by the non-narcotic entity and activation of opiate receptors by the narcotic entity.9 Narcotic combination drugs are among the most frequently prescribed drugs in the United States.10 A recent review of the top 200 prescribed drugs in the United States revealed that among all prescription drugs, acetaminophen plus hydrocodone was the most frequently prescribed.10 Acetaminophen combined with oxycodone, propoxyphene, or codeine was also on this list and ranked 25th, 56th, and 69th in overall prescriptions, respectively.10 A recently published paper revealed 85% of oral and maxillofacial surgeons almost always prescribe narcotic combination drugs for pain after dental impaction procedures.11 These combination analgesics provide proven analgesic efficacy, especially if the dose of the non-narcotic analgesic is in the 600-mg to 1000-mg range for acetaminophen and 200-mg to 400-mg range for ibuprofen.3-9 However, because of the narcotic component, analgesics often produce a relatively high incidence of side effects, including sedation, psychomotor impairment, nausea, and vomiting, and due to their direct effect of inhibiting intestinal peristalsis, constipation3,8,12 (Table 1).
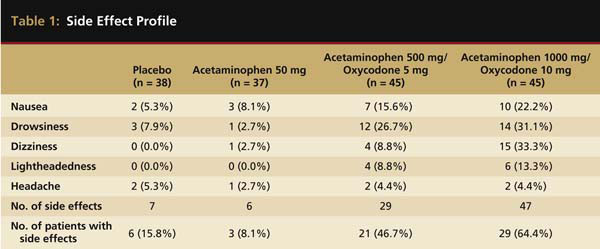
Side effect profile of placebo, acetaminophen 500 mg, acetaminophen 500 mg/oxycodone 5 mg, and acetaminophen 1000 mg/oxycodone 10 mg in patients receiving a single dose of the various drugs after dental impaction surgery. Table adapted from Cooper SA et al. Oral Surg Oral Med Oral Pathol. 1980;50(6):496-501.
With respect to postsurgical dental pain, many clinicians may overlook the fact that the non-narcotic is the agent providing the bulk of the analgesia whereas the narcotic entity is contributing to most of the side effects.3,12-14 Many studies have shown that with the dosages employed in dentistry, single-entity narcotics, such as 60 mg of codeine or 5 mg of oxycodone alone, are inferior to 500 mg of acetaminophen, 650 mg of aspirin, or 400 mg of ibuprofen. Often, they are only marginally superior to placebo in terms of analgesic effectiveness3,5,14 (Figure 1). Even 60 mg of immediate-release morphine is inferior to ibuprofen 400 mg for postsurgical dental pain.15 Highly efficacious narcotic analgesics with more favorable side effect profiles than those currently marketed would be welcomed additions to the dental postsurgical pain armamentarium.
Preclinical Experience with Tapentadol
Most side effects from narcotic analgesics are thought to be due to their binding to and activation of opioid receptors in the brain, spinal cord, and gastrointestinal tract. The development of a drug with less affinity for opioid receptors than classic narcotics, yet possessing an additional mechanism of action, was undertaken to create a compound with at least equivalent analgesic efficacy to narcotics but with better tolerability.1 The pharmacologic activity of tapentadol is due to both μ-opioid receptor stimulation and norepinephrine reuptake inhibition.2 Findings from animal models demonstrated tapentadol possessed approximately a 50-fold lower affinity for the rat μ-opioid receptor than did the prototype narcotic morphine. Yet it was only three times less potent as an analgesic. This suggested that tapentadol’s ability to increase synaptic concentrations of norepinephrine was also contributing to its analgesic effect.16 Results from animal model studies also proposed that the emetic effect of tapentadol was less than that of morphine.16
Clinical Acute Pain Studies with Tapentadol
Two acute-pain studies evaluating tapentadol have employed a bunionectomy model and compared tapentadol with other single-entity immediate-release narcotic formulations.17,18 Bunionectomy is considered an orthopedic procedure requiring osteotomy to correct a deformity and enlargement near the base of the first metatarsophalangeal joint, also called the hallux valgus.17 In addition to oral surgery pain, the bunionectomy pain model has been employed to study tapentadol and has many similarities to the widely employed impacted third molar pain model; when the local anesthesia abates, the trauma from the surgical procedure causes predictable pain levels of a moderate-to-severe intensity that persists for several days.19 The first clinical trial was a randomized, placebo-controlled, double-blind, multidose study in which patients took oral medications beginning the day after surgery. These included tapentadol 50 mg, tapentadol 100 mg, oxycodone 10 mg, or placebo every 4 to 6 hours for 3 days. Patients with inadequate pain relief were allowed to take 1000 mg of acetaminophen. If this was not effective, they could consume 400 mg of ibuprofen or 500 mg acetaminophen plus 5 mg of hydrocodone to remain in the study. All active treatments were more efficacious than placebo during both the first 24 hours and the full 72 hours post-treatment. In addition, while providing similar analgesic efficacy, tapentadol 50 mg was associated with lower rates of nausea (46.3% vs 71.6%), dizziness (32.8% vs 56.7%), vomiting (16.4% vs 38.8%), and constipation (6.0% vs 17.9%) than oxycodone 10 mg.17 In a similarly designed bunionectomy study, tapentadol 50 mg, 75 mg, and 100 mg, and oxycodone 15 mg were more efficacious than placebo.18 The incidence of nausea, vomiting, and constipation was 13%, 3%, and 1% in the placebo group; 35%, 18%, and 7% in the tapentadol 50-mg group; 38%, 21%, and 1% in the tapentadol 75-mg group; 49%, 32%, and 10% in the tapentadol 100-mg group; and 67%, 42%, and 15% in the oxycodone 15-mg group, respectively.18 The combined results of these two studies revealed that tapentadol appeared to have an efficacy equivalent to what would be considered high or supratherapeutic dosages of oxycodone compared with those routinely prescribed after dental surgery. Additionally the incidence of nausea, vomiting, and constipation appeared less with 50 mg to 100 mg of tapentadol compared with 10 mg to 15 mg of oxycodone. However, these side effects, especially nausea and vomiting, still occurred at a relatively high incidence with tapentadol over a 3-day period, certainly at a much greater rate than that reported with the use of non-steroidal anti-inflammatory drugs (NSAIDs). In a dental impaction pain multidose study in which patients could consume as much as six doses of 200 or 400 mg of ibuprofen for up to 7 days, the incidence of nausea was only 10.1% to 15.9% (compared with 35% to 49% for tapentadol in the bunionectomy pain studies) with no reports of vomiting or constipation.20
Tapentadol at 25 mg, 50 mg, 75 mg, 100 mg, and 200 mg has also been evaluated in the oral surgery pain model.15 This was a double-blind, randomized, single-dose, placebo-controlled study with immediate-release morphine sulfate 60 mg and ibuprofen 400 mg serving as the active controls. The oral surgery acute pain model, as originally described by Cooper and Beaver,21 is considered a pivotal model by the FDA for assessing new analgesic preparations. It has a proven assay sensitivity, with the ability to distinguish active from placebo medications, as well as less active analgesics from more active ones.22 In this study, all tapentadol doses of 50 mg or higher, in addition to morphine and ibuprofen, were significantly more efficacious than a placebo. However, ibuprofen of 400 mg displayed greater peak and overall analgesic scores and a quicker onset to meaningful pain relief than all FDA-approved dosages (50 mg, 75 mg, and 100 mg) of tapentadol or morphine 60 mg (Figure 2). With respect to the median onset of meaningful relief, even the highest approved dose of tapentadol (100 mg) had a relatively slow median onset time to meaningful pain relief of 3.9 hours compared with only 1.5 hours for ibuprofen 400 mg.15 The greater efficacy of ibuprofen is probably because the generation of prostaglandins from arachidonic acid via the cyclooxygenase (COX) enzyme system at the extraction site plays a key role in pain development following the surgical removal of impacted third molars.23 Ibuprofen as a member of the NSAID class is a potent COX inhibitor. At the 400-mg dose, the drug is a highly effective analgesic in postsurgical dental pain.5,7,14,24 Table 2 compares the incidences of the four most commonly reported side effects in this study—dizziness, nausea, vomiting, and drowsiness. While tapentadol produced reduced rates of dizziness (24% to 38%), nausea (10% to 22%), and vomiting (6% to 10%) than morphine (59%, 61%, and 59%, respectively), ibuprofen 400 mg was the best-tolerated drug with side effect incidences of 12%, 2%, and 2%, respectively.
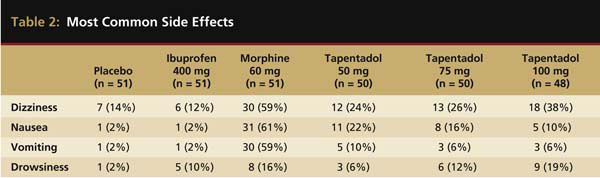
Most common side effects in the placebo, ibuprofen 400 mg, morphine 60 mg, and tapentadol 50 mg, 75 mg, and 100 mg groups. Table adapted from Kleinert R et al. Anesth Analg. 2008;107(6):2048-2055.
Recommendations
Nausea, vomiting, and constipation are common side effects that occur with analgesics containing narcotic entities. Tapentadol is an opioid with a dual mechanism of action. Clinical studies have revealed that compared to commonly employed narcotics, tapentadol appears to produce a lower incidence of nausea, vomiting, and constipation when compared to what would be considered high (oxycodone 10 mg) or supratherapeutic doses (oxycodone 15 mg and morphine sulfate 60 mg) of single-entity narcotics compared with doses employed in dental practice.15,17,18 Whether tapentadol produces a lower incidence of gastrointestinal side effects when compared with narcotic dosages typically employed for dental postoperative pain (hydrocodone 5 mg to 10 mg, codeine 60 mg, propoxyphene napsylate 100 mg, or oxycodone 5 mg)25 can be established only with additional research studies and/or clinical experience. Even assuming a better tolerability profile than other narcotic agents, tapentadol, like other single-entity narcotics, should not be used as a first-line drug for the treatment of postsurgical dental pain. A published dental impaction study showed that compared with ibuprofen 400 mg, tapentadol 50 mg to 100 mg was an inferior analgesic with respect to total and peak pain relief and onset of action.15
What then is the possible role of tapentadol in treating dental postoperative pain? While not specifically evaluated as of yet, tapentadol should provide an additive analgesic effect when combined with acetaminophen or NSAIDs, such as ibuprofen.3,5,7, 9,14 For breakthrough pain, despite a full analgesic dose of acetaminophen (650 mg to 1000 mg) or ibuprofen (400 mg to 600 mg), tapentadol 50 mg to 100 mg may be effective as “a rescue analgesic.” It appears that the 50-mg dose will be better tolerated than higher doses of the drug, although the trade-off will be somewhat less pain relief.15,17,18 Although tapentadol has less affinity for opiate receptors than classic narcotics,16 the FDA believes it has a high abuse potential, which could lead to severe physical and/or psychological dependence. Thus, tapentadol was placed in the Schedule II Category of Controlled Dangerous Substances (along with acetaminophen/oxycodone combinations and many other single-entity narcotics, such as morphine, hydromorphone, and oxycodone).26 Tapentadol should be prescribed in small quantities (no more than 10 tablets) and for a limited time. In general, refills written for acute pain medications, especially those containing an opioid, should be avoided. The package insert for tapentadol states that the recommended dosing schedule is every 4 to 6 hours. However, on the first day, a second dose may be taken as early as 1 hour after the first if adequate pain relief is not obtained.27 The daily dose should not exceed 600 mg.
Patients with impaired respiratory function (eg, severe bronchial asthma) or paralytic illeus, or those who have consumed monoamine oxidase inhibitors (MAOIs) within 14 days should not take tapentadol. In addition to MAOIs, warnings exist concerning the use of tapentadol in patients taking other antidepressants including tricyclic antidepressants (TCAs), serotonin selective reuptake inhibitors (SSRIs), and dual serotonin–norepinephrine reuptake inhibitors (SNRIs) because the combination may precipitate a serotonin syndrome (eg, tremors, convulsions, muscle rigidity, hyperexia) as well as more classic signs of opioid-induced central nervous system (CNS) depression.28 Similar to other narcotics, tapentadol impairs both mental and physical abilities, thus patients must be warned against operating motor vehicles and other hazardous machinery.27 Additive or supra-additive CNS depression is likely if tapentadol is consumed with other CNS depressants, such as alcohol, antihistamines, benzodiazepines, and other opioids.27 The clinician must read the full prescribing information.
Conclusions
Tapentadol is a recently approved narcotic analgesic, which also inhibits the neuronal reuptake of norepinephrine. Although nausea, vomiting, and constipation appear to occur at a lower incidence than relatively high oral doses of classic narcotics, these side effects still do occur. Tapentadol is less effective than ibuprofen 400 mg in treating postsurgical dental pain. It should not be used as a first-line analgesic for postsurgical dental pain but possibly as an “add-on” medication when optimal doses of ibuprofen or acetaminophen do not provide sufficient pain relief. Additional research is needed to study if tapentadol has any advantage over the narcotic entities and their dosages that are already employed in dentistry.
Disclosure
Dr. Hersh has received consulting fees from Ortho-McNeil, which manufactures tapentadol.
References
1. Wade WE, Spruill WJ. Tapentadol hydrochloride: a centrally acting oral analgesic. Clin Ther. 2009;31(12):2804-2818.
2. Tzschentke TM, Christoph T, Kögel B, et al. (-)-(1R,2R)-3-(3-dimethylamino-1-ethyl-2-methyl-propyl)-phenol hydrochloride (tapentadol HCl): a novel mu-opioid receptor agonist/norepinephrine reuptake inhibitor with broad-spectrum analgesic properties. J Pharmacol Exp Ther. 2007;323(1):265-276.
3. Cooper SA, Precheur H, Rauch D, et al. Evaluation of oxycodone and acetaminophen in treatment of postoperative dental pain. Oral Surg Oral Med Oral Pathol. 1980;50(6):496-501.
4. Cooper SA, Kupperman A. The analgesic efficacy of flurbiprofen compared to acetaminophen with codeine. J Clin Dent. 1991;2(3):70-74.
5. Van Dyke T, Litkowski LJ, Kiersch TA, et al. Combination oxycodone 5 mg/ibuprofen 400 mg for the treatment of postoperative pain: a double-blind, placebo- and active-controlled parallel-group study. Clin Ther. 2004;26(12):2003-2014.
6. Ziccardi VB, Desjardins PJ, Daly-DeJoy E, et al. Single-dose vicoprofen compared with acetaminophen with codeine and placebo in patients with acute postoperative pain after third molar extractions. J Oral Maxillofac Surg. 2000;58(6):622-628.
7. Wideman GL, Keffer M, Morris E, et al. Analgesic efficacy of a combination of hydrocodone with ibuprofen in postoperative pain. Clin Pharmacol Ther. 1999;65(1):66-76.
8. Litkowski LJ, Christensen SE, Adamson DN, et al. Analgesic efficacy and tolerability of oxycodone 5 mg/ibuprofen 400 mg compared with those of oxycodone 5 mg/acetaminophen 325 mg and hydrocodone 7.5 mg/acetaminophen 500 mg in patients with moderate to severe postoperative pain: a randomized, double-blind, placebo-controlled, single-dose, parallel-group study in a dental pain model. Clin Ther. 2005;27(4):418-429.
9. Beaver WT. Combination analgesics. Am J Med. 1984;77(3A):38-53.
10. Lamb E. Top 200 prescription drugs of 2008. Pharmacy Times. http://www.pharmacytimes.com/issue/pharmacy/2009/2009-05/RxFocusTop200Drugs-05092010. Accessed March 26, 2010.
11. Moore PA, Nahouraii HS, Zovko JG, et al. Dental therapeutic practice patterns in the U.S. II. Analgesics, corticosteroids, and antibiotics. Gen Dent. 2006;54(3):201-207.
12. Cooper SA. Narcotic analgesics in dental practice. Compend Contin Educ Dent. 1993;14(8):1061-1068.
13. Hargreaves KM, Troullos ES, Dionne RA. Pharmacologic rationale for the treatment of acute pain. Dent Clin North Am. 1987;31(4):675-694.
14. Cooper SA, Engel J, Ladov M, et al. Analgesic efficacy of an ibuprofen-codeine combination. Pharmacotherapy. 1982;2(3):162-167.
15. Kleinert R, Lange C, Steup A, et al. Single dose analgesic efficacy of tapentadol in postsurgical dental pain: the results of a randomized, double-blind, placebo-controlled study. Anesth Analg. 2008;107(6):2048-2055.
16. Tzschentke TM, de Vry J, Terlinden R, et al. Tapentadol HCl. Drugs Future. 2006;31:1053-1061.
17. Stegmann JU, Weber H, Steup A, et al. The efficacy and tolerability of multiple-dose tapentadol immediate release for the relief of acute pain following orthopedic (bunionectomy) surgery. Curr Med Res Opin. 2008;24(11):3185-3196.
18. Daniels SE, Upmalis D, Okamoto A, et al. A randomized, double-blind, phase III study comparing multiple doses of tapentadol IR, oxycodone IR, and placebo for postoperative (bunionectomy) pain. Curr Med Res Opin. 2009;25(3):765-776.
19. Desjardins PJ, Black PM, Daniels S, et al. A randomized controlled study comparing rofecoxib, diclofenac sodium, and placebo in post-bunionectomy pain. Curr Med Res Opin. 2004;20(10):1523-1537.
20. Hersh EV, Cooper S, Betts N, et al. Single dose and multidose analgesic study of ibuprofen and meclofenamate sodium after third molar surgery. Oral Surg Oral Med Oral Pathol. 1993;76(6):680-687.
21. Cooper SA, Beaver WT. A model to evaluate mild analgesics in oral surgery outpatients. Clin Pharmacol Ther. 1976;20(2):241-250.
22. Cooper SA. Single-dose analgesic studies: The upside and downside of assay sensitivity. In: Max MB, Portenoy RK, Laska EM eds. Advances in Pain Research and Therapy: The Design of Analgesic Clinical Trials. New York, NY: Raven Press; 1991:117-125.
23. Roszkowski MT, Swift JQ, Hargreaves KM. Effect of NSAID administration on tissue levels of immunoreactive prostaglandin E2, leukotriene B4, and (S)-flurbiprofen following extraction of impacted third molars. Pain. 1997;73(3):339-345.
24. Hersh EV, Levin LM, Cooper SA, et al. Ibuprofen liquigel for oral surgery pain. Clin Ther. 2000;22(11):1306-1318.
25. Hersh EV, Desjardins PJ, Trummel CL, et al. Non-opioid analgesics, nonsteroidal antiinflammatory drugs, and antirheumatic and antigout Drugs. In: Pharmacology and Therapeutics for Dentistry (ed. 6). Yagiela JA, Dowd FJ, Johnson B, et al, eds, Philadelphia, PA: Elsevier; 2010:324-358.
26. Drug Enforcement Administration Department of Justice. Schedules of controlled substances: placement of tapentadol into Schedule II. Fed Regist. 2009;74(97):23790-23793.
27. NUCYNTA™ (tapentadol) Immediate release oral tablets. Physicians Desk Reference. 64th ed. Montvale NJ: PDR Network LLC; 2010: 2643-2648.
28. Hersh EV, Pinto A, Moore PA. Adverse drug interactions involving common prescription and over-the-counter analgesic agents. Clin Ther. 2007;29(suppl):2477-2497.

